Test: Coordination Chemistry- 2 - Chemistry MCQ
30 Questions MCQ Test - Test: Coordination Chemistry- 2
The number of manganese ions in tetrahedral and octahedral sites, respectively in Mn3O4 are:
The spinels CoFe2O4 and FeFe2O4, respectively are:
In the trigonal bipyramidal crystal field, the d orbital with the highest energy is:
The magnetic moment of the complex K3[CoF6] is 5.0 μB . The total stabilization energy will be:
For the complexion [Cu(NH3)6]2+,the coordination geometry will be:
The correct statement about the Cu–N bond distance in [Cu(NH3)6]2+ is:
Which of the following shows NORMAL spinel structure:
CFSE of transition metal complexes can be determined by:
Which of the following pairs of electronic configuration of high–spin transition metal ions (3d) in an octahedral field undergo a substantial Jahn–Teller distortion:
According to the crystal field theory, Ni2+ can have two unpaired electron in:
The enthalpies of hydration of Ca2+, Mn2+, Zn2+ follow the order:
The correct order of acidity among the following species is:
The correct order of d-orbital splitting in a trigonal bipyramidal geo metry is:
It is known that pKa of water is 15.7. Based on this water pKa benchmark, arrange the fo llowing solvated metals–aqua io ns in order of their increasing acidity : Mn2+(H2O)6 , Fe3+(H2O)6, Cu2+(H2O)6 ,Ca2+(H2O)6
The pair of simple and inverse spinels respectively is:
Stabilization of highest oxidation states of transition metals by strong electronegative ligands due to:
The volume (in mL) of 0.1 M AgNO3 required for complex precipitation of chloride ions present in 30 mL of 0.01 M solution of [Cr(H2O)5Cl]Cl2, as silver chloride is close to
The number of donor sites in dimethyl glyoxime, glycinato, diethylene triamine and EDTA are respectively:
Among the complexes:
(I) K4[Fe(CN)6],
(II) K3[Co(CN)6],
(III) K4[Mn(CN)6], Jahn – Teller distortion is expected in.
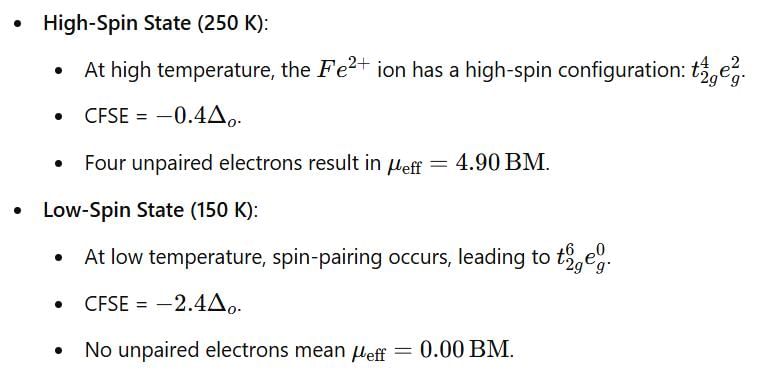
The complex [Fe(phen)2(NCS)2] (phen = 1,10–phenanthroline) shows spin cross–over behavior.CFSE and µeff at 250 and 150 K, respectively are:
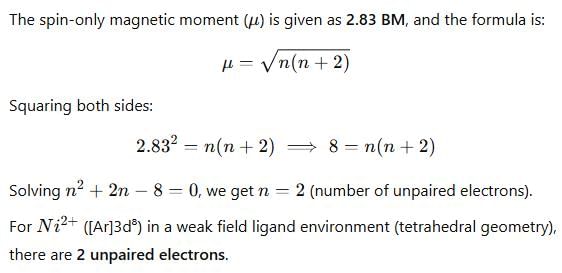
Given that the expected spin-only magnetic moment for (Et4N)2[NiCl4] is 2.83 BM, the total number of unpaired electrons in this complex is.
Of the following metal ions, which has the largest magnetic moment in its low-spin octahedral complexes?
The electronic configurations that have orbital angular momentum contribution in octahedral environment are: