Test: Organometallic Chemistry- 1 - Chemistry MCQ
20 Questions MCQ Test - Test: Organometallic Chemistry- 1
In Ziegler-Natta catalysis the commonly used catalyst system is:
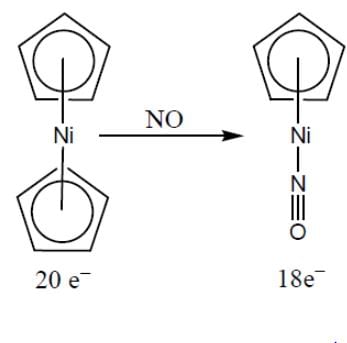
The substitution of η5-Cp group with nitric oxide is the easiest for:
Among the following, the unstable carbonyl species is:
The organometallic compound W(C5H5)2(CO)2 follows the 18-electron rule, The hapticities of the two cyclopentadienyl groups are:
Which of the following will exhibit optical isomerism:
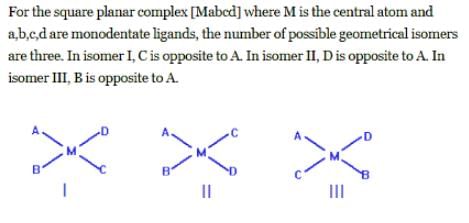
The compounds [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2 constitutes a pair of:
The order of CO bond strengths in the following metal hexacarbonyls is likely to be:
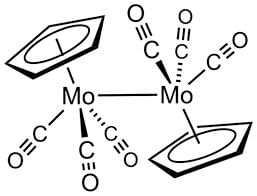
How many M — M bonds are present in [Cp Mo(CO3)]2?
Solid Co2(CO)8 shows infrared CO stretching bands at 1857, 1886, 2001, 2031, 2044, 2059, 2071 and 2112 cm–1. When Co2(CO)8 is dissolved in hexane, the carbonyl bands at 1857 and 1886 cm–1 disappear. These changes in the infrared spectrum in hexane are due to:
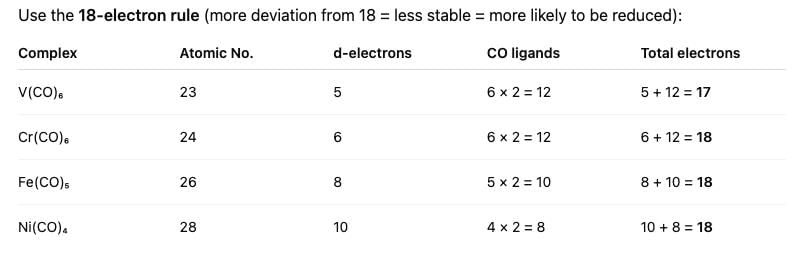
Which one of the following specie will be most easily reduced:
The final product in the reaction
[Mn (CO)6]- + MeLi → is :
In the complex, [Ni2(η5-Cp)2(CO)2] the IR stretching frequency appears at 1857 cm-1 (strong) and 1897 cm-1 (weak). The valence electron count and the nature of the M—CO bond respectively are:
Amongst the following, the metal that does not form homoleptic polynuclear metal carbonyl is:
Complexes of general formula, fac–[Mo(CO)3(Phosphine)3] have the C—O stretching frequency bands as given below,
Phosphines: PF3 (P), PCl3 (Q), P(Cl)Ph2 (R), PMe3(S)
ΝCO, cm–1 : 2090 (I); 2040 (II); 1977 (III); 1945 (IV)
What kind of isomerism is exhibited by octahedral [Co(NH3)4Br2]Cl:
In which of the following pairs, both the complexes show optical isomerism:
The ionization isomer of [Cr(H2O)4Cl(NO2)]Cl is :
Which of the following organometallic compound is σ and π bonded -