Test: Bio Inorganic- 1 - Chemistry MCQ
20 Questions MCQ Test - Test: Bio Inorganic- 1
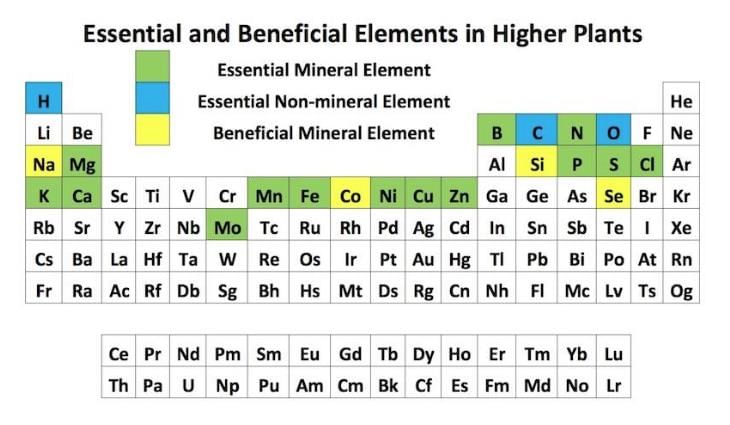
The correct set of the biologically essential elements, is ______.
In metal ion catalysis, what is the primary function of the metal ion?
High dose of dietary supplement ZnSO4 for the cure of Zn deficiency:
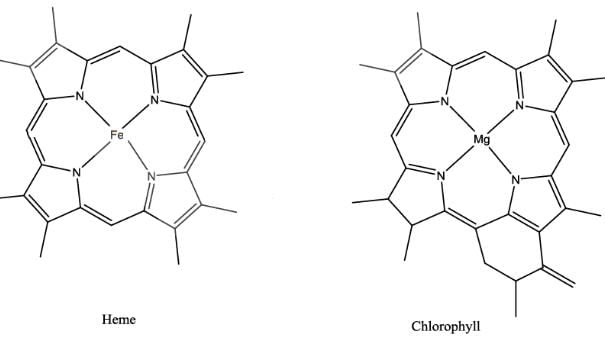
The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:
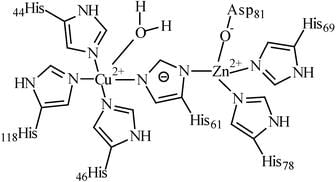
The number of histidine amino acid nitrogen atoms coordinated to bimetallic active site of oxyhemocyanin and oxyhemerythrine, respectively, are:
The biological functions of carbonic anhydrase and Carboxypeptidase A, respectively, are
The Fe—Npprphyrin bond distances in the deoxy and oxy-hemoglobin, respectively are:
The biological functions of the cytochrome P450 and myoglobin are, respectively:
The reduction of nitrogen to ammonia, carried out by the enzyme nitrogenase, needs:
In bacterial ruberdoxin, the number of iron atoms, sulfur bridges and cysteine ligands are:
A metal ion that replace manganese (II) ion in Mangano–proteins without changing its function, is:
The changes (from A–D given below) which occur when O2 binds to hemerythrin are:
(A) One ion atom is oxidized.
(B) Both the iron atoms are oxidized.
(C) O2 binds to one iron atom and is also hydrogen bonds.
(D) O2 binds to both the iron atoms and is also hydrogen bonded.
Amongst the following, the group that is bound to the metal ion in cenzyme B12 is:
Based on the behavior of the metalloenzymes, consider the following statements:
(A) In the enzymes, the zinc act ivates O2 to form peroxide species.
(B) In the enzymes, the zinc activates H2O and provides a zinc bound hydroxide.
(C) In the enzymes, the zinc activates O2 to break the bounding between the two oxygens.
(D) Zincion acts as a nucleophile and attacks at the peptide carbonyl.
The set of correct statements is,
Fe2+ -porphyrins fail to exhibit reversible oxygen transport and cannot differentiate CO from O2. However, the hemoglobin is free from both these pit falls. Among the following the correct set of statements is:
(A) Fe2+-porphyrins undergo μ-oxodimer formation and the same is prevented in case of the hemoglobin.
(B) Fe–CO bond strength is much low in case of hemoglobin when compared to the Fe2+ -porthyrins.
(C) While Fe–CO is linear, Fe–O2 is bent and is recognized by hemoglobin.
(D) The interlinked four monomeric units in the hemoglobin are responsible to overcome the pitfalls.