Test: Reaction Mechanism Level - 2 - Chemistry MCQ
30 Questions MCQ Test - Test: Reaction Mechanism Level - 2
When 2-chloro-2-methylbutane is refluxed with alcoholic KOH, the main product obtained is:
Using given codes, arrange the following compounds in decreasing order of the rate of solvolysis by SN1 mechanism:


1, 3-Dichloropropane one reaction with Zn and NaI gives:
In the following reactions:
(a)
(b)
(c)
(d)
The reaction intermediate formed will be
The intermediate in the reaction of m-bromoanisole with sodamide in liquid amnonia has:
Which of the alkyl halides undergoes most readily for nucleophilic substitution reaction:
The reaction of ethanolic KOH on 1, 1-dichloropropane gives:
Which reactive intermediate is believed to be part of the reaction shown:
In this transformat ion,
What is the best structure for A?
The major product formed in the following reaction is:
Which of the following statements does not fit in the criteria of E2 reactions?
The major product formed in the following reaction is:
The electrophilic aromatic substitution proceeds through an intermediate:
The relative rates of nitration of R – C6H5, where R = CH3, NO2, OH and Cl is:
Which of the following statements is not true for the E2 reactions?
Which of the following carbocations is the most stable?
Which of the following elimination reactions will give 1-butene as the major product?
Which of the following haloalkanes will undergo hydrolysis most readily?
Arrange the following three chlorides in decreasing order of SN1 reactivity
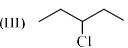
(I) CH3CH2CH2Cl
Arrange the following carbanions in order of their decreasing stability:
Arrange the compounds in order of decreasing reactively toward electrophilic substitution:
The major product expected from the following reactionis?
The rate of decarboxylation of isomeric carboxylic acids is:
An intermediate in racemization of (R)-3-phenyl-2-butanone is:
The intermediate involved in Curtius rearrangement is:
In the SN1 solvolysis of the following primary alky chlorides in aqueous ethanol, the order of decreasing reactivity is:














