Test: Pericyclic Level - 2 - Chemistry MCQ
30 Questions MCQ Test - Test: Pericyclic Level - 2
The reactivity of compound I-IV with maleic anhydride (V) follows the order:
Order of reactivity of the following dienes X, Y and Z in Diels-Alder reaction is:
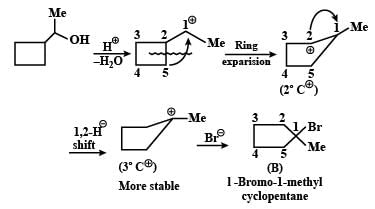
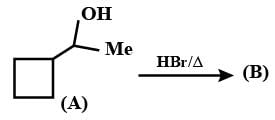
Give the major product and the mechanism involved in the following reaction.
Amongst the following the compound that does not act as a diene in Diels-Alder reaction is:
In the reaction of Cyclopentadiene with acrylate ester giving diels-Alder reaction products, the interacting frontier orbitals are:
Diels-Alder reaction normally yields endo-adduct as a major product. This is due to:
In the [4 + 2] cycloaddition of 1, 3-butadiene and ethylene:
The reaction of 1-bromo-2-fluorobenzene with furan in the presence of one equivalent to Mg gives
The diene which undergoes Diels–Alder reaction with maleic anhydride is:
In a Diels–Alder reaction, the most reactive diene amongst the following is: