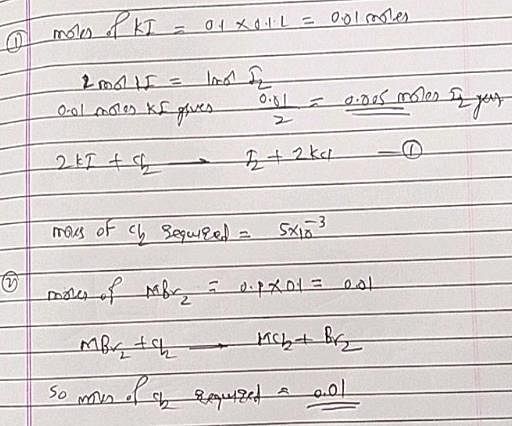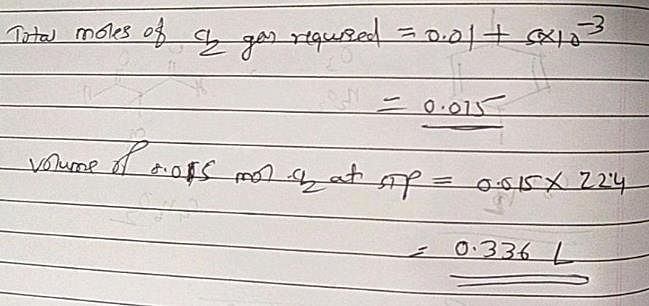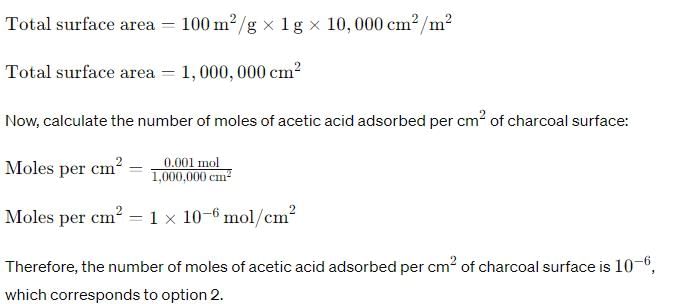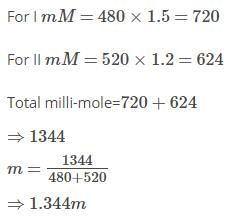Test: Mole Concept, Volumetric & Redox - 3 - Chemistry MCQ
30 Questions MCQ Test - Test: Mole Concept, Volumetric & Redox - 3
The total number of electrons in one molecule is carbon dioxide is
When the same amount of zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide, the ratio of volumes of hydrogen evolved is
2.76 g of silver carbonate on being strongly heated yields a residue weighing
If 0.5 mol of BaCl2 is mixed with 0.20 mol of Na3PO4, the maximum amount of Ba3(PO4)2 that can be formed is
A molal solution is one that contains one mole of solute in
Which of the following changes with increase in temperature:
How many moles of electron weighs one kilogram?
Given that the abundances of isotopes 54Fe, 56Fe and 57Fe are 5%, 90% and 5% respectively, the atomic mass of Fe is
Dissolving 120 g of urea (mol. Wt. 60) in 1000 g of water gave a solution of density 1.15 g/mL. The molarity of the solution is
The hydrated salt Na2CO3.x H2O undergoes 63% loss in mass on heating and becomes anhydrous. The value of x is
The volume of chlorine at STP required to liberate all the bromine and iodine in 100 mL of 0.1 M each of KI and MBr2 will be:
0.5400 g of a metal X yields 1.020 g of its oxide X2O3. The number of moles of X is:
Consider the reaction 2A + B + 3C → P + 2Q. Starting with 3 mol of A, 2 mol of B and 6 mol of C, number of moles of the products P and Q would respectively be:
A mixture of methane and ethane in the mole ratio of x : y has a mean molecular mass equal to 20. What would be the mean molecular mass of a mixture of the same gases present in the ratio y : x?
A partially dried clay mineral contains 8% water. The original sample contained 12% water and 45% silica. The percentage of silica in the partially dried sample is nearly
H2O2 in aqueous solution decomposes on warming as: 2H2O2(aq) → 2H2O(l) + O2(g) If 1 mole of gas occupies a volume of 25 L under the conditions of measurement and 200 ml of x M solution of H2O2 produces 5L of O2, the value of x is
5.0 g of a certain element X forms 10.0 g of its oxide having the formula X4O6. The atomic mass of X is
Charcoal (1 g) of surface area 100 m2 per gram, adsorbs 60 mg of acetic acid from an aqueous solution at 25°C and 1 atmosphere pressure. The number of moles of acetic acid adsorbed per cm2 of charcoal surface is:
The ionic strength of 0.1 M aqueous solution of Fe2(SO4)3 is:
In the following sequence of reactions, the overall yield (%) of O is:
Number of atoms in 558.5 g Fe (at. wt. 55.85) is:
In a compound C, H, N atoms are present in 9 : 1 : 3.5 by weight. Molecular weight of compound is 108. Its molecular formula is:
What volume of hydrogen gas at 273 K and 1 atm pressure will be consumed in obtaining 21.6 g of elemental boron (at. mass = 10.8) from the reduction of boron trichloride with hydrogen:
25 mL of a solution of barium hydroxide on titration with 0.1 molar solution of hydrochloric acid gave a titre value of 35 mL. The molarity of barium hydroxide is:
6.02 × 1020 molecules of urea are present in 100 mL solution. The concentration of urea solution is:
To neutralize completely 20 mL of 0.1 M aqueous solution of phosphorus (H3PO3) acid the volume of 0.1 M aqueous KOH solution required is:
Two solution of a substance (non-electrolyte) are mixed in the following manner,480ml of 1.5M of first solution with 520 ml of 1.2m of second solution.The molarity of final solution is
If 1/6, in place of 1/12, mass of carbon atom is taken to be the relative atomic mass unit, the mass of one mole of a substance will: