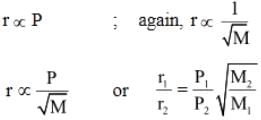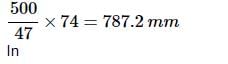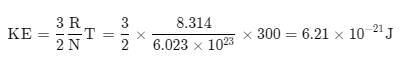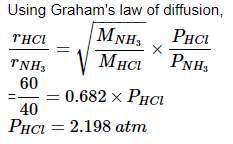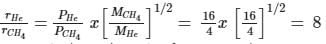Test: Gaseous State - 3 - Chemistry MCQ
30 Questions MCQ Test - Test: Gaseous State - 3
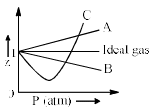
The given graph represent the variations of Z (compressibility factor 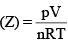 versus p, for three real gases A, B and C. Identify the only incorrect statement:
versus p, for three real gases A, B and C. Identify the only incorrect statement:
The root mean square speeds at STP for the gases H2, N2, O2 and HBr are in the order
Which of the following are same for all ideal gas at same STP?
4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:
3.7 g of a gas at 25°C occupied the same volume as 0.184 g of hydrogen at 17°C and at the same pressure. What is the molecular weight of the glass:
A hydrocarbon contains 10.5 g of carbon per gram of hydrogen. 1 L of the vapour of the hydrocarbon at 127°C and 1 atm pressure weights 2.8 g. Find the molecular formula of the hydrocarbon.
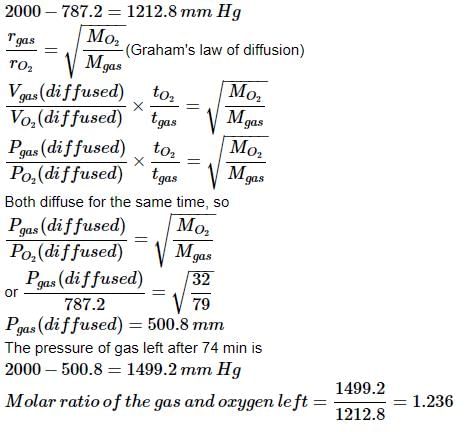
The pressure in a bulb dropped from 2000 to 1500 mm of mercury in 47 min when the contained oxygen leaked through a small hole. The bulb was then evacuated. A mixture of oxygen and another gas of molecular weight 79 in the molar ratio of 1 : 1 at a total pressure of 4000 mm of mercury was introduced. Find the molar ratio of the two gases remaining in the bulb after a period of 74 min.
Calculate the average kinetic energy, in Joule per molecule in 8.0 g of methane at 27°C:
At room temperature, ammonium gas at 1 atm pressure and hydrogen chloride gas at p atm pressure are allowed to effuse through identical pin holes from opposite ends of a glass tube of one metre length and of uniform cross-sect ion. Ammonium chloride is first formed at a distance of 60 cm from the end through which HCl gas is sent in. What is the value of p:
Calculate the pressure exerted by one mole of CO2 gas at 273 K, if the van der Waals’ constant ‘a’ = 3.592 dm6 atm mol–2. Assume that the volume occupied by CO2 molecules is negligible.
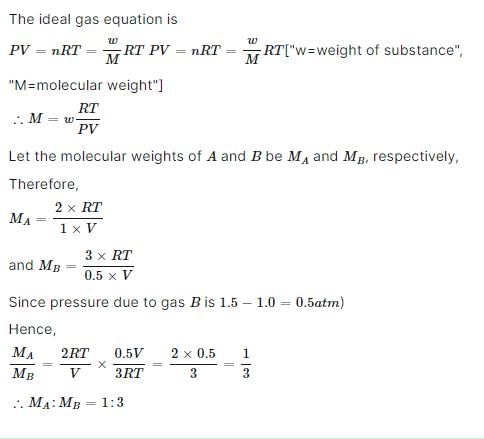
When 2 g of a gas A is introduced into an evacuated flask kept at 25°C, the pressure is found to be one atmosphere. If 3 g of another gas B is then added to the same flask, the total pressure becomes 1.5 atm. Assuming ideal gas behaviour, calculate the ratio of the molecule weights MA : MB.
Oxygen is present in one litre flask at a pressure of 7.6 × 10–10 mm Hg. Calculate the number of oxygen molecules in the flask at 0°C.
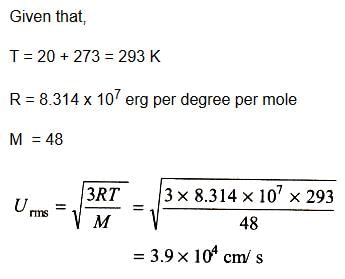
Calculate the root mean square velocity in cm/s of ozone kept in a closed vessel at 20°C and 82 cm mercury pressure.
A spherical balloon of 21 cm diameter is to be filled up with hydrogen at NTP from a cylinder containing the gas at 20 atmospheres at 27°C. If the cylinder can hold 2.82 L of water, calculate the number of balloons that can be filled up.
A 4 : 1 molar mixture of He and CH4 is contained in a vessel at 20 bar pressure. Due to a hole in the vessel, the gas mixture leaks out. What is the composition of the mixture effusing out initially.
At 27°C, hydrogen is leaked through a tiny hole into a vessel for 20 min. Another unknown gas at the same temperature and pressure as that of hydrogen is leaked through same bole for 20 min. After the effusion of the gases the mixture exerts a pressure of 6 atm. The hydrogen content of the mixture is 0.7 mole. If the volume of the container is 3 L. What is the molecular weight of the unknown gas?
A mixture of ethene (C2H6) and ethene (C2H4) occupies 40 L at 1.00 atm and at 400 K. The mixture reacts completely with 130 g of O2 to produces CO2 and H2O. Assuming ideal gas behaviour, Calculate the mole fractions of C2H4 and C2H6 in the mixture.
In the gas phase, the ratio of excluded volume to molecule volume for a spherical molecule is……………
In an ideal monoatomic gas, the speed of sound is given by . If the speed of sound in argon at 25°C us 1245 km h–1, the root mean square velocity in ms–1 is…………….
A stream of oxygen molecules at 500 K exists from a pin-hole in an over and strikes a slit that selects the molecules travelling in a specific direction. Given that the pressure outside the over is 2.5 × 10–7 atm, estimate the maximum distance at which the slit must be placed from the pin-hole in order to produce a collimated beam to oxygen, (Radius of O2 = 1.8 × 10–10 m).
The compression factor (compressibility factor) for one mole of a van der Waal’s at 0oC and 100 atmospheric pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible, calculate the van der Waals constant a.
At 400 K, the root mean square (rms) speed of a gas X (molecule weight = 40) is equal is equal to the most probable speed of gas Y at 60 K. The mo lecular weight of the gas Y is:
Using van der Waals’ equation, calculate the constant ‘a’ when two moles of a gas confined in a four litre flask exert a pressure of 11.0 atm at a temperature of 300 K. The value of ‘b’ is 0.05 L mol–1: