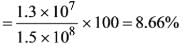GATE Chemical Engineering Mock Test - 6 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 6
The disorderly assembly insisted that the defendant be _____________ without undergoing a trial.
Which of the following conclusions can be drawn from the provided passage?
Certain students did not participate in the strike. If this statement is accurate, which of the following conclusions must logically follow?
- i. Some individuals who participated in the strike were students
- ii. No students participated in the strike
- iii. At least one student participated in the strike
- iv. Some individuals who did not participate in the strike were students
A merchant has sold six computer hardware items, the details of which are as follows:
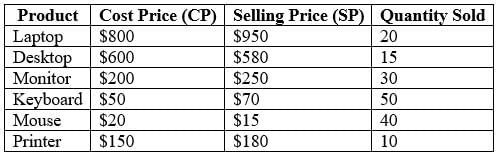
What is the overall profit or loss incurred from the sale of these six items?
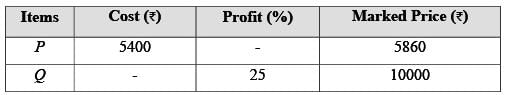
The table above shows the prices of two items, P and Q. The ratio of the price of item P to that of item Q is 3 : 4. The discount is defined as the difference between the marked price and the selling price. The profit percentage is determined by taking the ratio of the difference between the selling price and the cost price to the cost price.
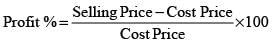
What is the discount on item Q expressed as a percentage of its marked price? ______.
In a class, the proportion of boys to girls is 7 to 3. From the choices listed below, which one represents a valid total number of students in the class?
John and Jane each conducted a thermodynamic experiment independently, where X and Y denote the initial and final thermodynamic states of the system, respectively. John carried out the experiment under reversible conditions, resulting in a change in entropy of the system of . Conversely, Jane executed her experiment under irreversible conditions, leading to a change in entropy of the system of . Which of the following statements is CORRECT?
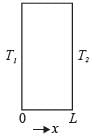
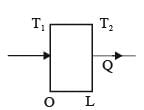
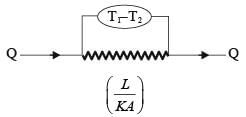
A slab with a thickness of L, depicted in the figure below, possesses a cross-sectional area A and a uniform thermal conductivity k. The temperatures at positions x=0 and x=L are represented as T1 and T2, respectively. Which of the following options provides the CORRECT formula for thermal resistance in the context of steady-state one-dimensional heat conduction?
If M₁ gm of a substance with specific gravity S₁ is mixed with M₂ gm of a substance with specific gravity S₂ and then the substances are used to make an alloy. What will be the specific gravity of the alloy?
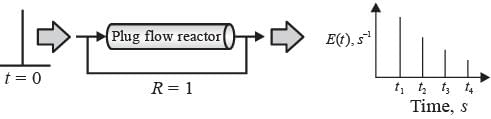
In a laboratory setting, a unit pulse input of tracer is introduced to an ideal plug flow reactor functioning at steady state with a recycle ratio, R = 1. The age distribution E(t) of the tracer at the reactor's outlet is recorded. The first four pulses detected at times t1, t2, t3, and t4 are illustrated below.
Additionally, please consider the following data and assumptions:
- R is defined as the ratio of the volume of fluid that is returned to the entrance of the reactor to the volume that leaves the system.
- No reactions take place within the reactor.
- Neglect any dead volume present in the recycle loop.
If the space time of the plug flow reactor is τ seconds, which of the following statements is accurate?
Given that 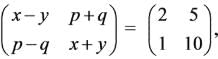 , what is the sum of x, y, p, and q?
, what is the sum of x, y, p, and q?
A straight fin with a uniform circular cross-section and an adiabatic tip has an aspect ratio (length/diameter) of 4. Given that the Biot number (calculated using the radius of the fin as the characteristic length) is 0.04, what is the fin efficiency in percentage terms? (round off to the nearest integer)
Consider a continuous flow of an incompressible, Newtonian fluid moving through a smooth circular pipe. Let αlaminar and αturbulent represent the kinetic energy correction factors for laminar and turbulent flow in the pipe, respectively. For the turbulent flow within the pipe,
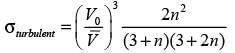
In this case, 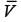 denotes the average velocity, V0 signifies the centerline velocity, and n is a parameter. The ratio of the average velocity to the centerline velocity for turbulent flow in the pipe is expressed as
denotes the average velocity, V0 signifies the centerline velocity, and n is a parameter. The ratio of the average velocity to the centerline velocity for turbulent flow in the pipe is expressed as
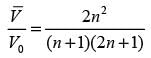
For n = 7, the value of  is _______. (round off to 2 decimal places)
is _______. (round off to 2 decimal places)
Formaldehyde is generated through the oxidation of methane within a reactor, where two parallel reactions take place.
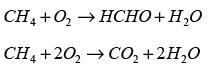
Methane and oxygen are introduced into the reactor, and the exit gases consist of methane, oxygen, formaldehyde, carbon dioxide, and water vapor. 60 mol s-1 of methane is supplied to the reactor. The molar flow rates (in mol s-1) for CH4, O2, and CO2 exiting the reactor are recorded as 26, 2, and 4 respectively. What is the molar flow rate of oxygen entering the reactor in _______ mol s-1?
Which of the following assertions regarding the thermodynamic characteristics of pure substances is accurate?
A settling experiment is conducted in an elongated column utilizing a dilute mixture that contains an equal quantity of particles of type A and type B dispersed in water (density 1000 kg m−3) at ambient temperature. The particles of type A are spherical with a diameter of 30 μm and a density of 1100 kg m−3, while type B particles are spherical with a diameter of 10 μm and a density of 1900 kg m−3. Assuming that Stoke's law is applicable throughout the experiment, what would be the characteristics of the settled bed?
Associate the reactions in Group-1 with their corresponding types in Group-2.
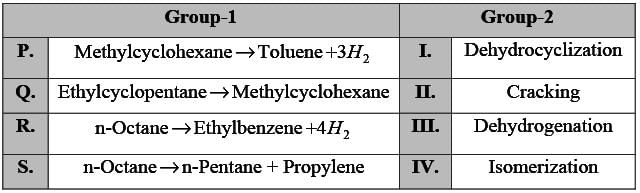
The appropriate pairs are:
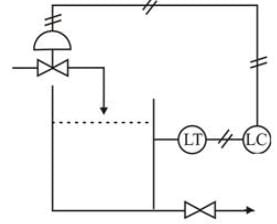
A liquid-level control system can be set up as depicted in the illustration, where a control valve regulates the flow of liquid into the storage tank. Considering that the liquid level transmitter is consistently direct acting, what type of control action should a proportional pneumatic controller exhibit if the control valve operates as air-to-open and air-to-close respectively?
In an extraction procedure, feed solution F is combined with solvent B. The carrier liquid present in the feed is A, while the solute is C. Below is the ternary diagram illustrating a single ideal stage extraction, where the dashed lines symbolize the tie-lines.
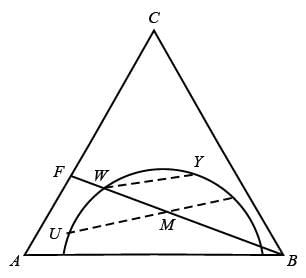
The CORRECT option(s) is/are
Which of the following statements is accurate concerning entropy and the Second Law of Thermodynamics?
What is the integer value of the first derivative of the function 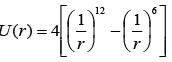 when evaluated at r = 1?
when evaluated at r = 1?
A gyratory crusher operates to reduce the feed size from 6 mm to 1 mm, consuming 43 kW.hr/tons of energy. If the target product size is modified from 1 mm to 0.50 mm using the same gyratory crusher, which involves further crushing the product already reduced to 1 mm diameter.
What will be the energy consumption, according to Bond's law?
(answer in kW.hr/tons up to two decimal places).
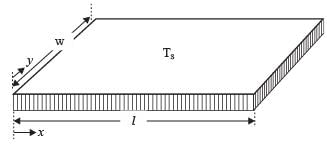
A fluid is flowing steadily under laminar conditions over a thin rectangular plate at temperature TS as shown in the figure below. The velocity and temperature of the free stream are u∞ and t∞, respectively. When the fluid flow is only in the x-direction, hx is the local heat transfer coefficient. Similarly, when the fluid flow is only in the y-direction, hy is the corresponding local heat transfer coefficient. Use the correlation Nu = 0.332 (Re)1/ 2 (Pr)1/3 for the local heat transfer coefficient, where, Nu, Re, and Pr, respectively are the appropriate Nusselt, Reynolds and Prandtl numbers. The average heat transfer coefficients are defined as 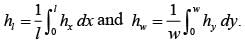 If w = 1 m and l = 4 m, the value of the ratio of
If w = 1 m and l = 4 m, the value of the ratio of 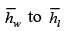 is _______ (in integer).
is _______ (in integer).
Air that is partially saturated at a pressure of 1 bar and a temperature of 50°C is brought into contact with water in an adiabatic saturator. The air undergoes cooling and humidification until it reaches saturation, exiting at a temperature of 25°C with an absolute humidity of 0.02 kg of water per kg of dry air. Consider the latent heat of vaporization of water to be 2450 kJ.kg-1, and the average specific heat capacities for dry air and water as 1.01 kJ.kg-1K-1 and 4.18 kJ.kg-1K-1, respectively. If the absolute humidity of the air entering the adiabatic saturator is H x 10-3 kg of water per kg of dry air, what is the value of H __________ (rounded off to two decimal places)?
Fresh catalyst is loaded into a reactor before the start of the following catalytic reaction.
A → products
The catalyst gets deactivated over time. The instantaneous activity a(t ), at time ��, is defined as the ratio of the rate of reaction 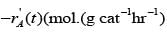 to the rate of reaction with fresh catalyst. Controlled experimental measurements led to an empirical correlation
to the rate of reaction with fresh catalyst. Controlled experimental measurements led to an empirical correlation 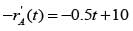
where t is in hours. The activity of the catalyst at t = 10 hr is _______ (rounded off to one decimal place).
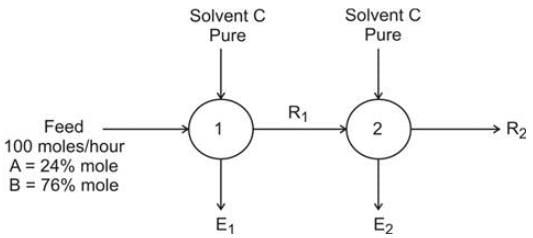
E1 and E2 represent the extracts; R1 and R2 signify the raffinates. An equal quantity of solvent is utilized in both stages 1 and 2.
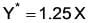
Determine the percentage recovery of A in the entire liquid-liquid extraction process. (To two decimal places in %)
A mixture comprising 40 mole% P and 60 mole% Q is subjected to distillation in a column with a feed rate of 160 kmol/hour, resulting in a liquid product that contains 30 mole% of P. What is the vapor product, in K mol/hour, given that the relative volatility is 4?________
In the chemical industry, a common guideline suggests that Rs.1 of annual sales necessitates an investment of Rs.2 in fixed capital. In a chemical processing facility where this guideline is applicable, the overall capital investment totals Rs. 1.5 x 108, while the working capital constitutes 20 percent of the total capital investment. The total annual product cost is Rs. 9.0 x 107. Given that the income tax rate on gross earnings is 35 percent, calculate the percentage of total capital investment that is returned annually as net profit _____________
The parallel reaction occurring in the liquid phase:

is carried out in an isothermal plug flow reactor. The initial concentration of A is 2.0 Kmol/m3. There are no products present in the feed. If the conversion of A reaches 80%, calculate the exit concentration of R (kmol/m3) __________
(up to two decimal places)
Carbon monoxide (CO) is combusted in the presence of a 200% excess of pure oxygen. The incoming streams are at 25ºC. The standard heat of formation (at 25ºC) for CO and CO2 are -110 kJ mol-1 and -390 kJ mol-1, respectively. The heat capacities (in J mol-1K-1) of the components are
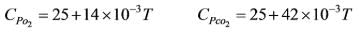
where T denotes the temperature in K. If the heat loss (in kJ) for each mole of CO combusted is 34.62, what is the flame temperature in K?



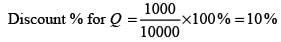
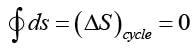
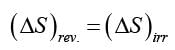
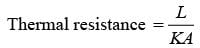
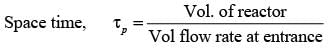
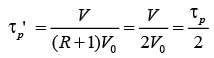
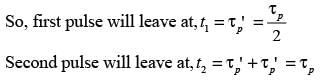
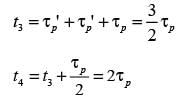
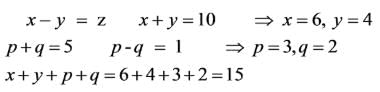
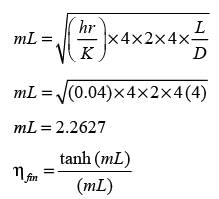

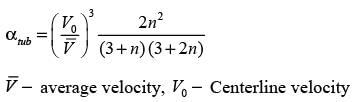
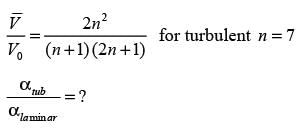
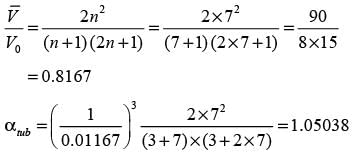
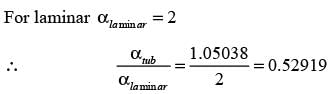
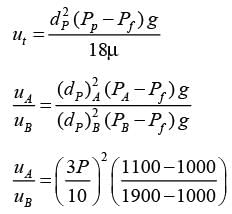
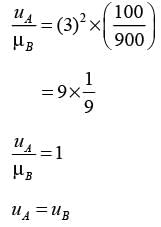
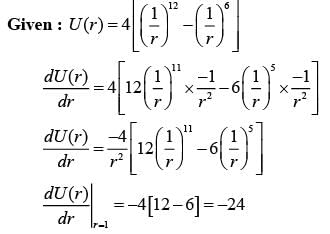

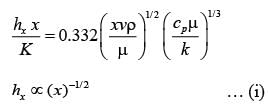
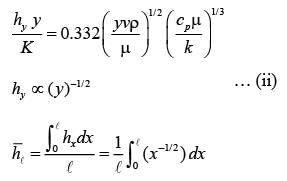
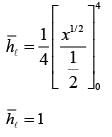
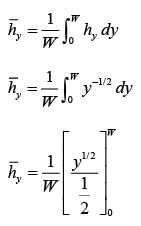
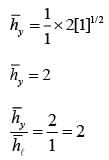

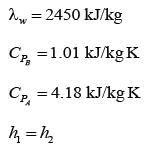
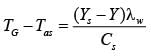
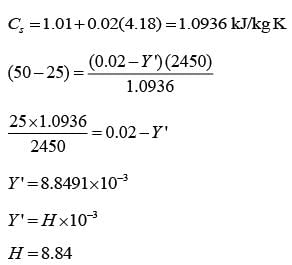
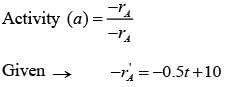
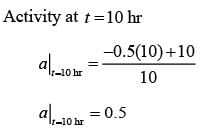
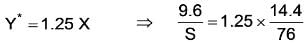
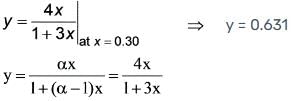
 D = 48.34 K mol/hour
D = 48.34 K mol/hour