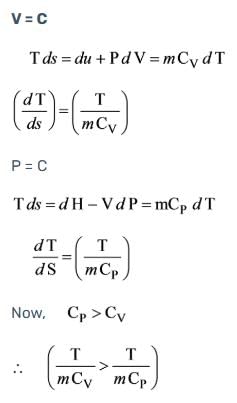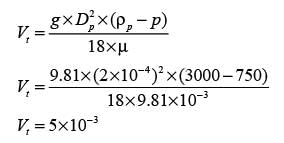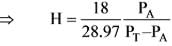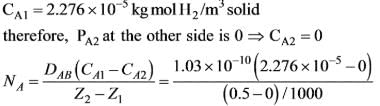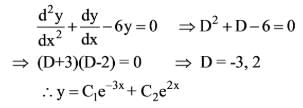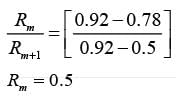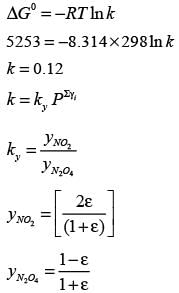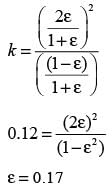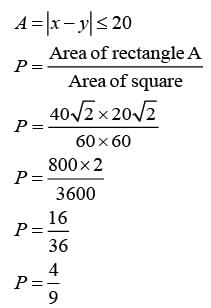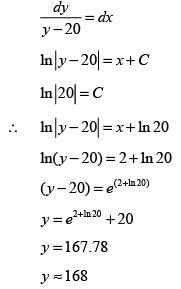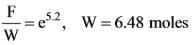GATE Chemical Engineering Mock Test - 10 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 10
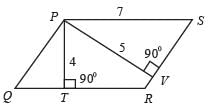
In the figure provided, PQRS is a parallelogram where PS measures 7 cm, PT is 4 cm, and PV is 5 cm. What is the measurement of RS in centimeters? (The diagram serves as a representation.)
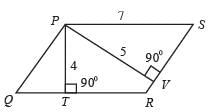
A circular piece of paper is folded according to the directions indicated in the image. Once the paper is punched in the final folded configuration as depicted and then unfolded in the opposite sequence of folding, it will appear as________.
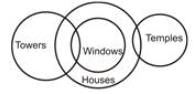
Assume the following statements to be true. Evaluate all the conclusions and select the option for which the conclusion logically follows.
Statements:
Some towers are windows.
All windows are houses.
Some houses are temples.
Conclusions:
I. Some towers are temples.
II. Some houses are towers.
III. Some temples are windows.
Statement 1: Synthesis gases invariably consist of both CO and H2.
Statement 2: Not all synthesis gases contain H2.
Which of the following statements is accurate?
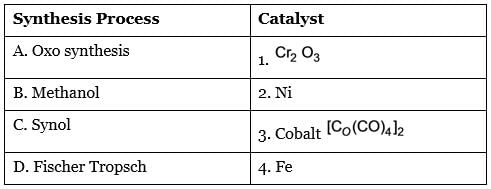
Associate the synthesis methods with their corresponding required catalysts.
What are the primary applications of essential oils?
The concept of wet bulb temperature revolves around the balance between the rates of energy transfer to the ______ and the process of ______ evaporation.
The change from laminar to turbulent flow in natural convection on vertical plates takes place when the product of the Prandtl number and the Grashof number is approximately equal to
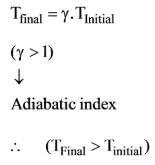
What is the slope of the constant volume line for an ideal gas represented on a T-s diagram?
A rigid, adiabatic tank that has been evacuated is gradually filled with air from a supply line that delivers air at a steady pressure PL and temperature TL. What will be the temperature of the air in the tank once the filling process is complete?
A solid spherical particle is subjected to free settling in a fluid with a density of 750 kg m−3 and a viscosity of 9.81 x 10−3 Pa s. The density of the particle is 3000 kg m−3, and its diameter measures 2 x 10−4 m. The acceleration due to gravity is 9.81 m s−2. Assuming that Stokes' law applies, what is the terminal settling velocity (in m s−1) of the particle?
When two identical first order systems are connected in series, the resulting system will be a ___________ 2nd order system.
In relation to the production of ammonia gas, the method is designated based on specific pressure and temperature conditions.
Match the following:

Considering that E (in W.m-2) represents the total hemispherical emissive power of a surface kept at a specific temperature, which of the following statements is/are CORRECT?
A manufacturing operation for a plant entails the production of liquid Mono ethylene Glycol utilizing gaseous oxygen and ethylene as feedstock. Given that the cost of the delivered equipment for the plant is approximately 75 lakh, what would be the ratio of fixed capital investments to the total capital investment? (Rounded to three decimal places) Assume: The long multiplication factor is applicable. ……………….
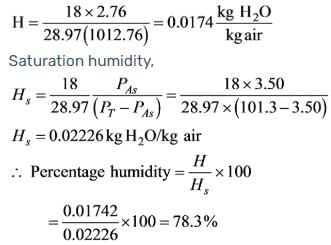
The temperature of the air in a room is 26.7ºC with a pressure of 101.325 kPa. This air has water vapour contributing to a partial pressure of PA = 2.76 kPa. At 26.7ºC, the vapour pressure of water vapour is 3.50 kPa, and the molecular weight of air is 28.97. What is the percentage of humidity present?
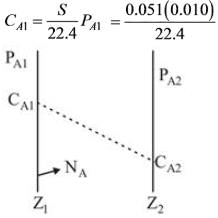
Hydrogen gas at a temperature of 17ºC and a partial pressure of 0.010 atm is diffusing through a membrane made of vulcanized neoprene rubber that is 0.5 mm thick. The pressure on the opposite side of the neoprene is zero. The solubility of H2 gas in neoprene at 17ºC is 0.051 m3 (at standard temperature and pressure of 0° C and 1 atm) per m3 solid-atm, and the diffusivity DAB is 1.03 x 10-10 m2 / sec at 17ºC. What will be the flux at steady state through the membrane expressed in kmol H2 / m2 -sec, assuming that the membrane is the only barrier to diffusion?
What is the solution to the ordinary differential equation represented by the following image?
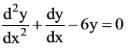
A shell-and-tube heat exchanger with a heating surface area of 10 m2 was acquired for Rs. 2.5 x 105 in 1990. The purchased cost capacity exponent is 0.60 for surface areas between 10 and 40 m2 and 0.81 for those ranging from 40 to 200 m2. Given that the cost index in 2000 is 1098 and in 1990 is 930, what will be the cost (in rupees) of a heat exchanger with 100 m2 of heating surface in the year 2000? _________
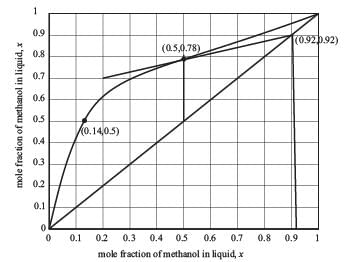
Examine the equilibrium data for the methanol-water system at 1 bar presented in the figure below.
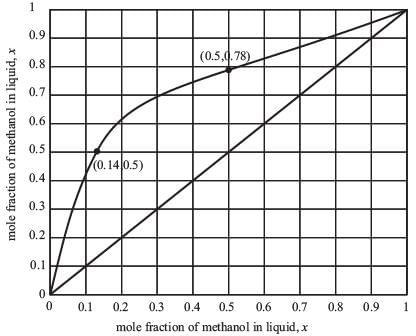
A distillation column functioning at 1.0 bar is tasked with producing 92 mol % methanol. The feed is a saturated liquid and consists of an equimolar mixture of methanol and water. What is the minimum reflux ratio?
Examine the gas phase reaction N2O4 ⇔ 2NO2 taking place in an isothermal and isobaric reactor held at 298 K and 1.0 bar. The standard Gibbs energy change for this reaction at 298 K is 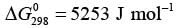 . The standard states refer to pure ideal gases at 1.0 bar. The equilibrium mixture in the reactor behaves like an ideal gas. The universal gas constant is 8.314 J mol−1K−1. If one mole of pure N2O4 is initially introduced into the reactor, what fraction of N2O4 decomposes into NO2 at equilibrium?
. The standard states refer to pure ideal gases at 1.0 bar. The equilibrium mixture in the reactor behaves like an ideal gas. The universal gas constant is 8.314 J mol−1K−1. If one mole of pure N2O4 is initially introduced into the reactor, what fraction of N2O4 decomposes into NO2 at equilibrium?
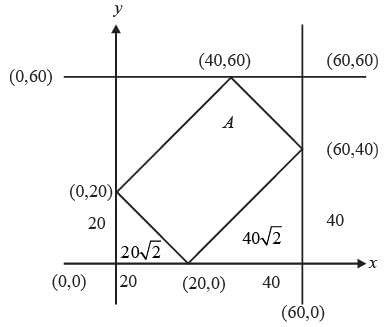
An exhibition took place in a hall on 15 August 2022 from 3 PM to 4 PM, during which any individual was permitted to enter only a single time. Attendees who entered prior to 3:40 PM left the hall precisely 20 minutes after their entry time. Those who entered at or after 3:40 PM exited exactly at 4 PM. The arrival time of any visitor is uniformly distributed between 3 PM and 4 PM. Two individuals, X and Y, entered the exhibition hall independently of one another. What is the probability that their visits to the exhibition overlapped?
If dy/dx = y - 20 and y|x=0 = 40, what is the value of y when x = 2? (round off to the nearest integer).
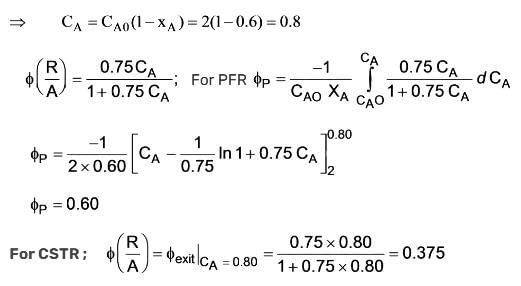
Initially, a concentration of 2 mol/litre of substances A and B was used. The overall yield is calculated for a conversion rate of 60%. What will the overall yield be if a CSTR is utilized? (Round your answer to three decimal places) ……….

A gas phase reaction of second order is occurring in a plug flow reactor (PFR), represented as P → 2Q. A conversion rate of 50% is sought, with P entering the reactor at a concentration of 15 mol/L. The volumetric flow rate is 2 L/sec, and the rate constant is 4 L/mol.s. The change in density is neglected in the volume calculations. What will be the percentage error in the volume calculation? ________
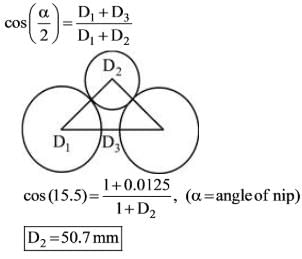
In the case of crushing rolls with a diameter of 1 m, set with a gap of 12.5 mm between the crushing surfaces and an angle of nip measuring 31°, what is the maximum size (in mm) of the particle that can be fed into the rolls?
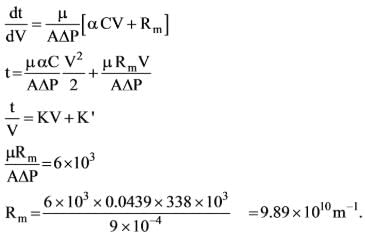
Data regarding the laboratory filtration of CaCO3 slurry in water at a temperature of 298.2 K and a constant pressure difference of 338 kPa is illustrated below.
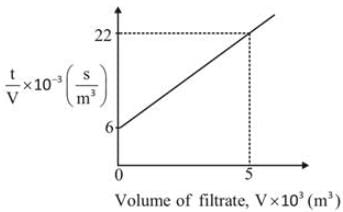
The filter area for the plate and frame press was A = 0.0439 m2 and the concentration of the slurry was Cs = 23.47 kg/m3. Determine the medium resistance ____________ x 1010 m-1
Data: The viscosity of water at 298.2 K is 9 x 10-4 Pa-sec. (up to two decimal places)
A batch process involves the differential distillation of 1175 moles of a monoethylene glycol-water mixture at a pressure of 45 kPa. The resulting data is illustrated in the graph below:
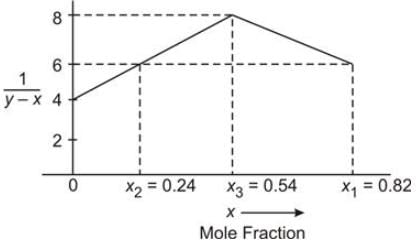
In this context:
x and y denote the mole fraction of monoethylene glycol in the liquid phase and the equilibrium vapor phase, respectively.
Here, x1 represents the composition of the feed, while x2 indicates the composition of the liquid final product in the still.
Determine the average composition of the distillate collected.
(Provide your answer up to two decimal places). ……………………………..
A proposal suggests utilizing solar energy to heat a sizable collector plate. The energy collected would subsequently be transferred as heat to a fluid within a heat engine, which would then release energy as heat into the atmosphere. Experiments show that approximately 1880 kJ/m2h of energy can be harvested when the plate operates at 90º C. Calculate the minimum area of the collector necessary for a facility generating 1 kW of useful shaft power, assuming the atmospheric temperature is 20º C.