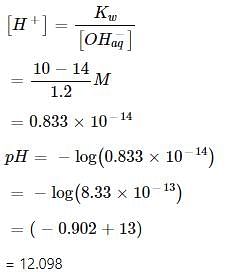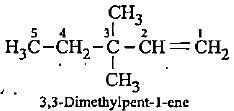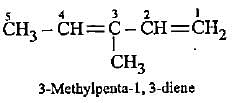Chemistry: Topic-wise Test- 3 - NEET MCQ
30 Questions MCQ Test - Chemistry: Topic-wise Test- 3
0.3 g of Ca(OH)2 is dissolved in water to give 500 mL of solution. The pH of the solution is
In order to decide what course the reaction adopts and make a qualitative prediction about the effect of a change in conditions on equilibrium we use
Acidity of BF3can be explained on the basis of which of the following concepts?
Spontaneity in the context of chemical thermodynamics means
Enthalpy of combustion of carbon to CO2is –393.5 kJ mol−1. Calculate the heat released upon formation of 35.2 g of CO2from carbon and dioxygen gas.
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Arrange the following compounds in increasing order of polarity
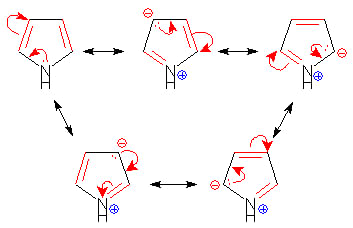
In principle, what is true regarding benzene and 1, 3, 5-cyclohexatriene?
Enthalpy of hydrogenation of cyclohexene is -119 kJ/mol and that of benzene is -208 kJ/mol. Based on these information, resonance energy of benzene can be calculated to be
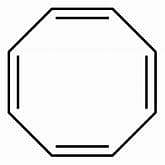
Which is not true regarding 1, 3, 5, 7-cyclooctatetraene?
How many minimum no. of C-atoms are required for position & geometrical isomerism in alkene?
How many structural formula are possible when one of the hydrogen is replaced by a chlorine atom in anthracene?
The number of cis-trans isomer possible for the following compound:
The number of isomers of dibromoderivative of an alkene (molear mass 186 g mol-1) is
Which among the following does not exhibit geometric isomerism?
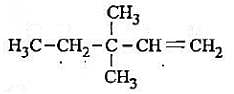
Which of the following represents 3-methylpenta-1, 3-diene?
Direction (Q. Nos. 11-14) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. For an ideal gas, consider only (p -V) work in going from initial state X to the final state Z. The final state Z can be reached either of the two paths shown in the figure. Which of the following choice (s) is (are) correct?
(Take ΔS as change in entropy and W as work done)
[IIT JEE 2012]
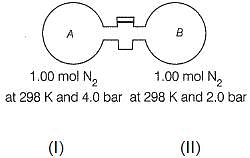
Passage II
The stopcock connecting A and B is of negligible volume. Stopcock is opened and gases are allowed to mix isothermally.
Q. Final pressure set up is
Consider the following process
Select correct choices(s)