JEE Main Chemistry Test- 1 - JEE MCQ
25 Questions MCQ Test - JEE Main Chemistry Test- 1
20 g of an ideal gas contains only atoms of S and O occupies 5.6 L at NPT. What is the mol. wt. of gas ?
5.6 litres of a gas at N.T.P. are found to have a mass of 11 g. The molecular mass of the gas is
A crystalline salt Na2SO4.xH2O on heating losses 55.9 per cent of its mass. The formula of crystalline salt is---
Vapour density of a metal chloride is 66. Its oxide contains 53% metal. The atomic weight of the metal is
When two aqueous solutions react together to give off solid product, this reaction is known as
At S.T.P. the density of CCl4 vapour in g/L will be nearest to
Which of the following transitions of electrons in the hydrogen, atom will emit maximum energy ?
The uncertainty found from the uncertainty principle (Δx.Δp = h/4 π) is
The spectrum of He is expected to be similar to that of
The electrons present in K-shell of the atom will differ in
Which of the following statements about quantum numbers is wrong?
Out of the two compounds shown below, the vapour pressing of B at a particular temperature is expected to be
In which of the following molecules, the central atom does not use sP3-hybrid orbitals in its bonding ?
From which of the following species it is easiest to remove one electron ?
Which element has the greatest tendency to lose electrons ?
0.01 mole of iodoform (CHI3) reacts with Ag to produce a gas whose volume at NTP in ml is :-
2CHI3 + 6Ag —→ 6AgI(s) + C2H2(g)
How many maximum electrons can be accomodate in 7th shell of element X?



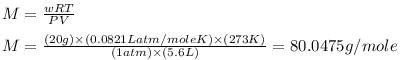 Therefore, the molar mass of the gas is, 80.0475 g/mole
Therefore, the molar mass of the gas is, 80.0475 g/mole