(24-01-2018) Major Test - 2 (GATE, Chemistry) - IIT JAM MCQ
30 Questions MCQ Test - (24-01-2018) Major Test - 2 (GATE, Chemistry)
In the presence of an external magnetic field (normal Zeeman effect), the Transition 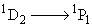 splits into:
splits into:
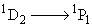 splits into:
splits into:In pyrsoulphuric acid (H2S2O5) oxidation number of both sulfers:
The energy of an electron in the first Bohr orbit of H-atom –13.6 eV the possible charge value(s) of excited states for electron in Bohr orbitals of hydrogen is:
For an endothermic reaction where DH represents the enthalpy of the reaction the minimum value for the energy of activation will be:
The edge length of a cube is 400 pm. Its body diagonal would be:
Partition function for a two level-system the lower state being non-degenerate the upper state is doubly degenerate:
Half-life of a third order reaction 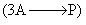 is given by:
is given by:
At room temperature, which molecule has the minimum rotational entropy?
The energy levels of the harmonic oscillator (neglecting zero point energy) are εv = nhν for
n = 0, 1, 2,…………  . Assuming hν = KBT, The partition function is:
. Assuming hν = KBT, The partition function is:
The properties like melting point, solubility, color, etc changes on varying the
Arrange the following increasing order of their acidic strength:
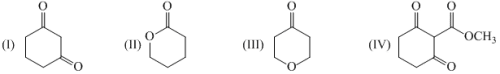
The number of chemical shift non-equilibrium protons in 1H NMR spectrum of α-pinene is:
Among the complexes (A) K4[Cr(CN)6], (B) K4[Fe(CN)6], (C) K3[Co(CN)6], and K4[Mn(CN)6]
John-Teller distortion is expected in:
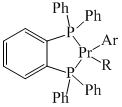
The reductive elimination of Ar—R (Coupled product) from A is facile when:
Isothermal which has fractional coverage, linearly, dependent on pressure at law pressure but almost independent at high pressure is called:
In cyclophesphazenes (NPX2) [X = F, Cl, Br and Me), the strength of P—N  varies with X in the order:
varies with X in the order:
Amongst the following, the metal carbonyl species having the highest νCO stretching frequency is:
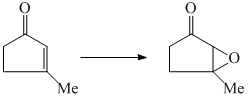
The suitable reagent for the following conversion is:
Among the following which is antiaromatic:
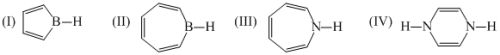
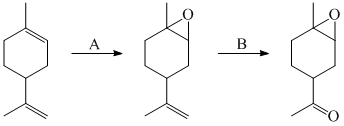
Reagent (A) and (B) in blow reaction are:
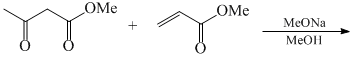
Carbonyl compounds can generally be converted to hydrocarbons by:
The first excited state of hydrogen molecule is:
In the absence of bound globin chain heme group on exposure to O2 gives the iron-oxygen species:
The major product formed in the following reaction is:
The structure type shape of the pelyhedrol (Skeletal) famework of the carborane, Me2C2B10H10, respectively, are:
Among the following, the natural product that is a steroid and contains an α, β-unsaturated lactone is:
HX is a weak acid (Ka = 10–5). If forms a salt NaX (0.1M) on reacting with caustic soda. The degree of hydrolysis of NaX is:
For the reaction;
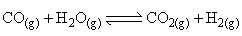
At a given temperature, the equilibrium amount of CO2(g) can be increased by














