Class XI (PCB) - Online Scholarship Test - MCQ
30 Questions MCQ Test - Class XI (PCB) - Online Scholarship Test
Acceleration of a particle under projectile motion at the highest point of its trajectory is :
Work done by all forces on a system of particles is equal to
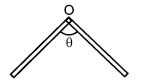
A thin rod of length L and mass M is bend at the middle point O as shown in figure. Consider an axis passing through the middle point O and perpendicular to the plane of the bent rod. Then moment of inertia about this axis is:
Three containers of same base area, same height are filled with three different liquids of same mass as shown in the figure. If F1, F2, F3 are the force exerted by the liquid on the base of the container in case I, II and III respectively, then we have the relation:
Temperature at which Fahrenheit and kelvin pair of scales give the same reading will be:
Two springs are in a series combination and are attached to a block of mass m which is in equilibrium. The spring constants and the extensions in the springs are as shown in the figure. Then the force exerted by the spring on the block is :
A cubical block of side a and density ρ slides over a fixed inclined plane with constant velocity v . There is a thin film of viscous fluid of thickness t between the plane and the
block . Then the coefficient of viscosity of the thin film will be : (Acceleration due to gravity is g)
Figure shows the velocity time graph of a particle moving along straight line (v is m/s and t is in seconds). Its average velocity in 20 seconds will be :
In a cyclic process ABCA for an ideal gas. In AB, BC and CA process 50 J, 20 J and 5 J heat is supplied to an ideal gas. In process AB internal energy of gas increases by 60 J and in process BC work done by gas is 30 J. The increase in internal energy of gas in process CA is :
Consider the following statements :
(i) Young's modulus is numerically equal to the stress which will double the length of a wire
(ii) Viscosity of gases is greater than that of liquids.
(iii) The surface tension of a liquid decreases due to the presence of insoluble contamination. The number of above statements that are true is
A massless conical flask filled with a liquid is kept on a table in a vacuum. the force exerted by the liquid on the base of the flask is W1. The force exerted by the flask on the table is W2.
The ratio of period of oscillation of the conical pendulum to that of the simple pendulum is. (Assume the strings are of the same length in the two cases and θ is the angle made by the string with the vertical in case of conical pendulum.)
A system of uniform cylinders and plates is shown in figure. All the cylinders are identical and there is no slipping at any contact. Velocity of lower & upper plate is V and 2V respectively as shown in figure. Then the ratio of angular speed of the upper cylinders to lower cylinders is
S1, S2 are two coherent sources (having initial phase difference zero) of sound located alongx-axis separated by 4 λ where λ is wavelength of sound emitted by them. Number of maxima located on the elliptical boundary around it will be :
The Vander waal equation for 1 mole of a real gas is (V – b) = RT where P is the pressure, V is the volume, T is the absolute temperature,R is the molar gas constant and a, b are Vander waal constants. The dimensions of a are the same as those of
Which of the following statements is not a correct statement about the trends when going from left to right across the periods of peridic Table -
Which of the following electronic configurations indicates the biggest jump between the second and third ionisation energy values ?
Maximum number of electrons that can be filled in M-shell of an atom is
Which of the following element has highest Ist ionisation potential :
An atom of an element has 11 protons and has a mass number 23. The nucleus of this atom contains .......... neutrons.
Number of bonds present in N2 molecule :
Which oil is used as frother in froth floatation process ?
Which of the following contains both ionic and covalent bond ?
500 ml vessel contains 1.5 M each of A, B, C and D at equilibrium. If 0.5 moles each of C and D are taken out, the value of Kc for A B C D will be : (at constant temperature)
X ml of H2 gas effuses through a hole in a container in 5 seconds. The time taken for the effusion of the same volume of the gas specified below under identical conditions is :
Which of the following are function of mesosome














