DCE CEE Previous Year Paper 2009 - JEE MCQ
30 Questions MCQ Test - DCE CEE Previous Year Paper 2009
3 moles of A and 4 moles of B are mixed together and allowed to come into equilibrium according to the following reaction: A(g) + 4B(g) 2C(g) + 3D(g) when equilibrium is reached, there is 1 mole of C. The equilibrium extent of the reaction is:
Which is not correct statement about the chemistry of 3d and 4f series elements ?
Bauxite ore is made up of Al2O3 + SiO2 + TiO2 + Fe2O3. This ore is treated with conc. NaOH solution at 500K and 35 bar pressure for few hours and filtered hot. In the filtrate the species present are:
Given the following sequence of reaction
The major product ‘C’ is :
Which is not the correct order for the stated property?
For square planar complex of platinum (II), [Pt(NH3)(Br)(Cl)Py] 0 , how many isomeric forms are possible ?
In pyrophosphoric acid, H4P2O7, number of σ-and dπ-pπ bonds respectively:
A 5% solution of sugarcane (Mol wt = 342) is isotonic with 1% solution of X under similar conditions. The mol. wt of X is:
For a reaction between A and B, the initial rate of reaction is measured for various initial concentration of A and B. The data provided are:
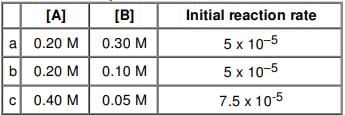
The overall order of the reaction is:
SF2, SF4 and SF6 have the hybridisations at sulphur atom respectively as:
For AB bond if percent ionic character is plotted against electronegativity difference (XA – XB). The shape of the curve would look like:
The correct curve is:
If the various terms in the below given expressions have usual meanings, the Van’t Hoff factor (i) cannot be calculated by which one of the following expressions:
In which pair of species, both species do have the similar geometry ?
In which reaction there will be increase in entropy?
For the reaction A → B ; ∆H = +24kJ / mole. For the reaction B → C ; ∆H = -18kJ / mole. The decreasing order of enthalpy of A, B, C follows the order:
Freundlich equation for adsorption of gases (in amount of X g) on a solid (in amount of m g) at constant temperature can be expressed as:
The number of isomeric pentyl alcohols possible are :
If liquid is dispersed in solid medium, then this is called as:
In forming (i) N2 → N2 + and (ii) O2→ O2 + ; the electrons respectively are removed from
For the given complex [CoCl2(en)(NH3)2] +, the number of geometrical isomers, the number of optical isomers and total number of isomers of all type possible respectively are:
The following two reactions of HNO3 with Zn are given as (Equations are not balanced) Zn + conc.HNO3 → Zn(NO3)2 + X + H2O (A) Zn + dilute.HNO3 → Zn(NO3)2 + Y + H2O (B) In reactions A and B, the compounds X and Y respectively are:
Small quantities of solution of compounds TX, TY and TZ are put into separate test tubes containing X, Y and Z solution. TX does not react with any of these. TY reacts with both X and Z. TZ reacts with X. The decreasing order of case of oxidation of the anions X – , Y – , Z – is:
When copper pyrites is roasted in excess of air, a mixture of CuO + FeO is formed. FeO is present as impurities. This can be removed as slag during reduction of CuO. The flux added to form slag is:
If k1 = Rate constant at temperature T1 and k2 = Rate constant at temperature T2 for a first order reaction, then which of the following relations is correct? (Ea : activation energy)
In the reaction for dinitration
The major dinitrated product ‘X’ is :
The number of unpaired electrons in gaseous species of Mn 3+, Cr 3+ and V 3+ respectively are............and most stable species is ............
For a chemical reaction, ∆G will always be negative if :




















