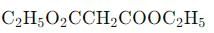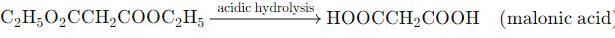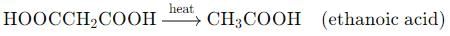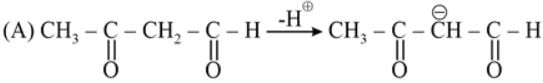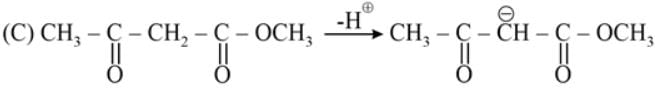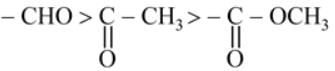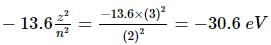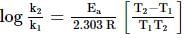VITEEE PCME Mock Test - 5 - JEE MCQ
30 Questions MCQ Test - VITEEE PCME Mock Test - 5
A function f(x) is differentiable at x = c(c  R). Let g(x) = |f(x)|, f(c) = 0 then
R). Let g(x) = |f(x)|, f(c) = 0 then
If ABCD is a rhombus whose diagonals cut at the origin O, then  +
+  +
+  +
+  equals
equals
 +
+  +
+  +
+  equals
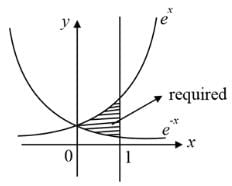
equalsThe area of the region bounded by the curves y = ex and y = e(-x) from x = 0 to x = 1 is:
Let A = {1, 2, 3}, B = {1, 3, 5}. A relation R: A → B is defined by R = {(1, 3), (1, 5), (2, 1)}. Then R⁻¹ is defined by:
Let θ ∈ [-2π,2π] and 2 cos2θ + 3 sin θ = 0 then sum of all solutions is
Power gain for N-P-N transistor is 106, input resistance 100Ω and output resistance 10000Ω. Find current gain.
At what distance from his face, a person should place concave mirror of focal length 0.4 m so that magnification is 5 times for a virtual image?
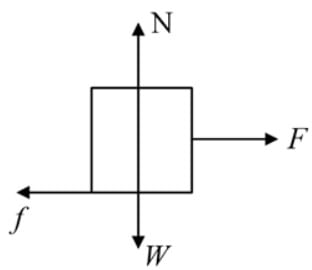
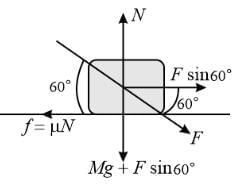
A weight W rests on a rough horizontal plane. If the angle of friction be θ, the least horizontal force that will move the body along the plane will be
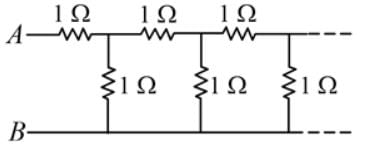
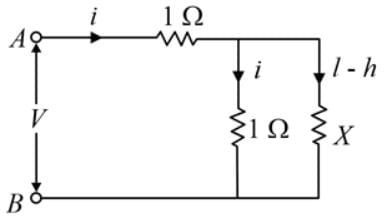
The equivalent resistance between the points A and B of an infinite network of resistances, each of 1 Ω, connected as shown is
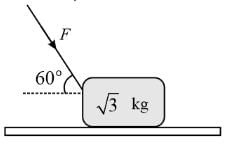
A force F is applied to a block of mass √3 kg resting on a horizontal surface with a coefficient of friction 1/√3. The maximum value of force F so that the block does not move is to be determined. (Take g = 10 m/s²).
A force F is required to break a wire of length l and radius r. What force is required to break a wire, of the same material, having twice the length and six times the radius?
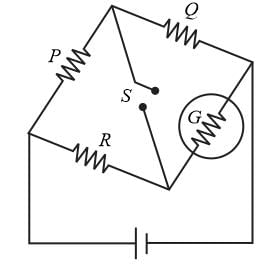
The figure shows a circuit diagram of a Wheatstone bridge to measure the resistance G of the galvanometer. The relation P/Q = R/G will be satisfied only when
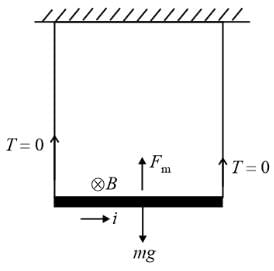
A conduction rod of 1 m length and 1 kg mass is suspended by two vertical wires through its ends. An external magnetic field of 2 T is applied normal to the rod. Now the current to be passed through the rod so as to make the tension in the wires zero is
[Take g = 10 ms−2]
The temperature of an iron block is 140°F. Its temperature on the Celsius scale is
The half-life period and the mean-life period of a radioactive element are denoted respectively by Th and Tm. Then
Calculate the emf of the cell in which the following reaction takes place.
Ni (s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag (s)
(Given: = 1.05 V)
Which of the following reagents is/are used in the Hinsberg test of amines?
Acidic hydrolysis of diethylmalonate, followed by heating, will yield which of the following carboxylic acids?
Which of the following statements is/are true?
P. N3- has larger ionic radius than O2-.
Q. Cr has higher atomic radius than Co.
R. Technetium has only two stable isotopes.
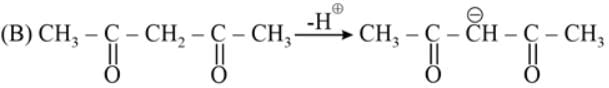
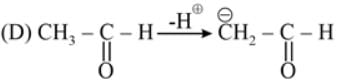
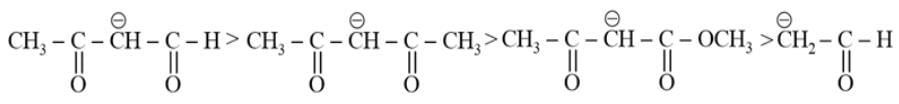
The most acidic α -hydrogen is in the following compound:
Zr and Hf have almost equal atomic and ionic radii because
According to Bohr's theory the energy required to remove electron from n = 2 of Li+2 ion is (given that the ground state ionization energy of hydrogen atom is 13.6 eV)
Activation energy of a chemical reaction can be determined by
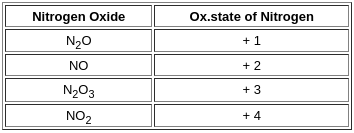
Arrange in the increasing order of oxidation state of nitrogen for following nitrogen oxides.
N2O, NO2, NO, N2O3
Read the following sentences carefully and choose the most appropriate phrase to join them.
I. Studies on nonlinear dependence on applied voltage can be expanded to more complex quantum transport experiments.
II. Experiments on artificial atoms are called quantum dots.
i. Ever since…
ii. Even though…
iii. For instance…
Each of the following questions has two sentences A and B.
Mark (A) if you think sentence A has an error.
Mark (B) if you think sentence B has an error.
Mark (C) if you think both sentences A and B have errors.
Mark (D) you think neither sentence has an error.
A. Because he wanted it done right, he always did it himself.
B. One criteria that is invariably used is your score in the written test.
The following sentence has a word or group of words missing. Four or five alternative words are given. You have to find out which one them would make the sentence grammatically correct and meaningful.
I left home "_____" a walk in the garden.





 f'(c)
f'(c)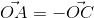 and
and 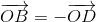
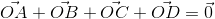

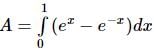
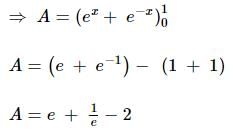
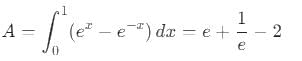



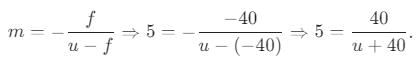
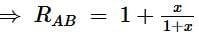
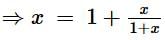
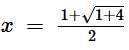
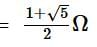
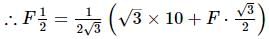
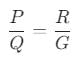
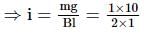
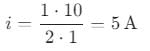
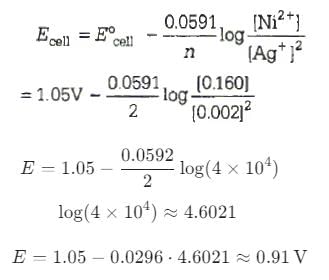
 R-N-Na+-SO2C6H5 (Soluble)
R-N-Na+-SO2C6H5 (Soluble) No reaction (Insoluble)
No reaction (Insoluble)