Test: Planck’s Law - Chemical Engineering MCQ
10 Questions MCQ Test - Test: Planck’s Law
The energy emitted by a black surface should not vary in accordance with
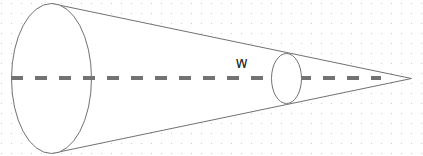
In the given diagram let r be the length of the line of propagation between the radiating and the incident surfaces. What is the value of solid angle W?
Likewise the amount of emitted radiation is strongly influenced by the wavelength even if temperature of the body is
A small body has a total emissive power of 4.5 kW/m2. Determine the wavelength of emission maximum
The sun emits maximum radiation of 0.52 micron meter. Assuming the sun to be a black body, Calculate the emissive ability of the sun’s surface at that temperature
The law governing the distribution of radiant energy over wavelength for a black body at fixed temperature is referred to as
A furnace emits radiation at 2000 K. Treating it as a black body radiation, calculate the monochromatic radiant flux density at 1 micron m wavelength
A metal sphere of surface area 0.0225 m2 is in an evacuated enclosure whose walls are held at a very low temperature. Electric current is passed through resistors imbedded in the sphere causing electrical energy to be dissipated at the rate of 75 W. If the sphere surfaces temperature is measured to be 560 K, while in steady state, calculate emissivity of the sphere surface



















