Test: Fick’s Law - Chemical Engineering MCQ
20 Questions MCQ Test - Test: Fick’s Law
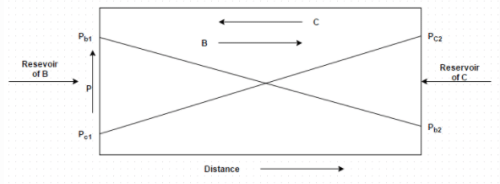
Figure shows equimolal diffusion between species B and C. Identify the correct molar diffusion rate
Estimate the diffusion coefficient for ammonia in air t 25 degree Celsius temperature and one atmospheric pressure.
For ammonia,
Molecular weight = 17
Molecular volume = 25.81 cm3/gm mole
For air,
Molecular weight = 29
Estimate the diffusion coefficient of carbon monoxide through air in which the mole fractions of each constituents are
O 2 = 0.18
N 2 = 0.72
CO = 0.10
The gas mixture is at 300 K and 2 atmosphere total pressure. The diffusivity values are
D CO = 18.5 * 10 -6 m2/s at 273 K, 1 atm
D CN = 19.2 * 10 -6 m2/s at 288 K, 1 atm
The subscripts C is carbon monoxide, O is oxygen and N is nitrogen
In the expression for molar flux NA=(NA+NB)CA/C – DABdCA/dz, the terms representing bulk flow and molecular diffusion are, respectively
Consider loss of ethanol vapor by diffusion from a half-filled open test tube. At what point in the diffusion path will the contribution of the bulk flow term to the molar flux be maximum?
A reaction 3A+2B+C=1.5D+E occurs in a reactor at steady state. What will be the value of the flux ratio NA/ND
For a component A of a mixture, concentration as a function of x is given:
CA=5e-10x (x is in cm and CA in mol/liter)
Calculate the value of diffusion velocity (m/s)of the component A at the point x=0, if diffusivity of A in the mixture is 2.567*10-5m2/s.
A sheet of Fe 1.0 mm thick is exposed to a oxidizing gas on one side and a deoxidizing gas on the other at 725°C. After reaching steady state, the Fe membrane is exposed to room temperature, and the C concentrations at each side of the membrane are 0.012 and 0.075 wt%. Calculate the diffusion coefficient (m2/sec) if the diffusion flux is 1.4×10-8kg/m2-sec.
At which condition molar flux with respect to a stationary observer and with respect to a observer moving with molar average velocity?
In industries titanium is hardened through diffusion of carbon. The concentration of carbon at 1mm into the surface of the titanium slab is 0.25kg/m3 and at 3mm the concentration is 0.68kg/m3. The rate at which the carbon is entering into its surface is 1.27*10-9kg/m2.s. calculate the value of diffusion coefficient of carbon.














