|
|
Akash KulkarniEduRev Chemistry |

|
Akash Kulkarni
EduRev Chemistry
|
Content, tests & courses saved by you for accessing later. (visible to you only)
Top Scoring Tests by Akash
Thermodynamic Potential MSQ | 16/20 |
Logic Gates MSQ | 16/20 |
Crystals – MSQ | 16/20 |
Statistical Physics MSQ | 16/20 |
IIT JAM Mathematics Practice Test- 13 | 68/90 |
IIT JAM Mathematics Practice Test- 14 | 68/90 |
Organic Chemistry MCQ | 25/35 |
IIT JAM Mathematics Practice Test- 11 | 35/50 |
Semiconductor Diode - MSQ | 14/20 |
IIT JAM Mathematics Practice Test- 2 | 35/50 |
Discussed Questions

|
Akash Kulkarni upvoted • Feb 27, 2025 |
The absorption at λmax 279 nm (ε = 15) in the UV spectrum of acetone is due to:
- a)π-π* transition
- b)σ-σ* transition
- c)n-π* transition
- d)π-σ* transition
Correct answer is option 'C'. Can you explain this answer?
The absorption at λmax 279 nm (ε = 15) in the UV spectrum of acetone is due to:
a)
π-π* transition
b)
σ-σ* transition
c)
n-π* transition
d)
π-σ* transition

|
Edurev.iitjam answered |
The type of transition is responsible for a band at λmax = 279 nm in absorption spectrum of acetone. In case of n → π* transitions, the polar solvents form hydrogen bonds with the ground state of polar molecules more readily than with their excited states. Therefore, in polar solvents the energies of electronic transitions are increased.

|
Akash Kulkarni upvoted • Feb 11, 2025 |
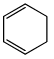
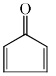
Which of the following molecules, in pure form, is (are) unstable at room temperature:- a)
- b)

- c)
- d)
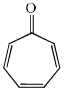
Correct answer is option 'B,C'. Can you explain this answer?
Which of the following molecules, in pure form, is (are) unstable at room temperature:
a)
b)
c)
d)

|
Asf Institute answered |
Correct Answer :- b,c
Explanation : (B) Cyclobutadiene follows Huckel's criteria for anti-aromaticity [(4n)−π electrons], hence unstable.
(C) Follows Huckel's criteria for anti-aromaticity [(4n)−π electrons], hence, unstable.

|
Akash Kulkarni upvoted • Feb 05, 2025 |
In radical chain polymerization, the quantity given by the rate of monomer depletion, divided by the rate of propagating radical formation is called:
- a)Kinetic chain length.
- b)Propagation efficiency.
- c)Propagation rate constant
- d)Polymerization time.
Correct answer is option 'A'. Can you explain this answer?
In radical chain polymerization, the quantity given by the rate of monomer depletion, divided by the rate of propagating radical formation is called:
a)
Kinetic chain length.
b)
Propagation efficiency.
c)
Propagation rate constant
d)
Polymerization time.

|
Edurev.iitjam answered |

Extent rate of reaction ?

|
Akash Kulkarni answered • Feb 01, 2025 |
Extent of Rate of Reaction
The extent of the rate of reaction refers to how quickly a chemical reaction occurs, which can be influenced by various factors. Understanding this concept is essential in chemistry, especially in both industrial applications and laboratory settings.
Factors Affecting Rate of Reaction
- Concentration: Increasing the concentration of re... more
The extent of the rate of reaction refers to how quickly a chemical reaction occurs, which can be influenced by various factors. Understanding this concept is essential in chemistry, especially in both industrial applications and laboratory settings.
Factors Affecting Rate of Reaction
- Concentration: Increasing the concentration of re... more
During the bacterial DNA replication, simultaneous synthesis of both the leading and lagging strand are catalyzed by “P”, and during translation, peptidyl transferase reaction is catalyzed by “Q”. Choose the correct combination of P and Q.- a)P – homodimer of DNA polymerase III Q – 16S rRNA
- b)P – asymmetric dimer of DNA polymerase III Q – 23S rRNA
- c)P – monomer of DNA polymerase III Q – 5S rRNA
- d)P – asymmetric dimer of DNA polymerase III Q – ribosomal proteins
Correct answer is option 'B'. Can you explain this answer?
During the bacterial DNA replication, simultaneous synthesis of both the leading and lagging strand are catalyzed by “P”, and during translation, peptidyl transferase reaction is catalyzed by “Q”. Choose the correct combination of P and Q.
a)
P – homodimer of DNA polymerase III Q – 16S rRNA
b)
P – asymmetric dimer of DNA polymerase III Q – 23S rRNA
c)
P – monomer of DNA polymerase III Q – 5S rRNA
d)
P – asymmetric dimer of DNA polymerase III Q – ribosomal proteins

|
Akash Kulkarni answered • Feb 01, 2025 |
Introduction
In bacterial DNA replication and translation, specific enzymes and ribosomal components play crucial roles. Understanding the functions of these molecules helps clarify the correct combination of P and Q in the question.
P: Leading and Lagging Strand Synthesis
- The synthesis of both leading and lagging strands during bacterial DNA replication is catalyzed... more
In bacterial DNA replication and translation, specific enzymes and ribosomal components play crucial roles. Understanding the functions of these molecules helps clarify the correct combination of P and Q in the question.
P: Leading and Lagging Strand Synthesis
- The synthesis of both leading and lagging strands during bacterial DNA replication is catalyzed... more

|
Akash Kulkarni upvoted • Jan 31, 2025 |
The acidity for the following compounds increases in the order:CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH- a) I < II < III
- b) II < III < I
- c) III < II < I
- d) II < I < III
Correct answer is option 'C'. Can you explain this answer?
The acidity for the following compounds increases in the order:
CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH
a)
I < II < III
b)
II < III < I
c)
III < II < I
d)
II < I < III

|
Edurev.iitjam answered |
a. EWG ( e−− withdrawing groups) increases the acidic strength, whereas EDG ( e−− donating groups) decrease the acidic strength.
b. Nearer is the EWG to the source [(−COOH)group], stronger is the acid, i.e., α− substituted halo acid stronger than β−orγ− substituted halo acid.
Increasing order of acidic strength:
(III)<(II)<(I).
Increasing order of acidic strength:
(III)<(II)<(I).

|
Akash Kulkarni upvoted • Jan 28, 2025 |
For the reaction in equilibrium, A 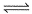 B
B
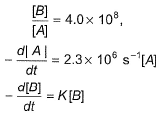
Thus, K is- a)
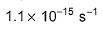
- b)

- c)
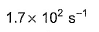
- d)
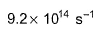
Correct answer is option 'B'. Can you explain this answer?
For the reaction in equilibrium, A 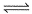 B
B
Thus, K is
Thus, K is
a)
b)
c)
d)

|
Sushil Kumar answered |
From the reaction
-d[A]/dt = d[B] /dt
⇒2.3 × 106 [A] = k [B]
as given in question [B] /[A] = 4 × 108
so [A] / [B] = 1/ 4 ×108
⇒ 2.3 × 106 . [A] /[B] = k
⇒ 2.3 × 106 / 4 × 108 = k
Or k = 5.8 × 10-3 /sec¹
-d[A]/dt = d[B] /dt
⇒2.3 × 106 [A] = k [B]
as given in question [B] /[A] = 4 × 108
so [A] / [B] = 1/ 4 ×108
⇒ 2.3 × 106 . [A] /[B] = k
⇒ 2.3 × 106 / 4 × 108 = k
Or k = 5.8 × 10-3 /sec¹
Equivalent conductivity of Fe2(SO4)3 is related to molar conductivity by the expression:- a)Λeq=Λm
- b)Λeq=Λm/3
- c)Λeq=3Λm
- d)Λeq=Λm/6
Correct answer is option 'D'. Can you explain this answer?
Equivalent conductivity of Fe2(SO4)3 is related to molar conductivity by the expression:
a)
Λeq=Λm
b)
Λeq=Λm/3
c)
Λeq=3Λm
d)
Λeq=Λm/6

|
Akash Kulkarni answered • Jan 09, 2025 |
Understanding Equivalent Conductivity
Equivalent conductivity (Λeq) is a measure of a solution's ability to conduct electricity, specifically per equivalent concentration of the solute. For ionic compounds like Fe2(SO4)3, we can relate it to molar conductivity (Λm) using the number of ions produced in solution.
Breaking Down Fe2(SO4)3
- Dissociation in Water:... more
Equivalent conductivity (Λeq) is a measure of a solution's ability to conduct electricity, specifically per equivalent concentration of the solute. For ionic compounds like Fe2(SO4)3, we can relate it to molar conductivity (Λm) using the number of ions produced in solution.
Breaking Down Fe2(SO4)3
- Dissociation in Water:
Which of the following changes would cause a shift in the membrane potential of a neuronal cell from –70 mV to –50 mV?- a)A decrease in K+ permeability
- b)An increase in Na+ permeability
- c)An increase in K+ permeability
- d)A decrease in Na+ permeability
Correct answer is option 'A,B'. Can you explain this answer?
Which of the following changes would cause a shift in the membrane potential of a neuronal cell from –70 mV to –50 mV?
a)
A decrease in K+ permeability
b)
An increase in Na+ permeability
c)
An increase in K+ permeability
d)
A decrease in Na+ permeability

|
Akash Kulkarni answered • Nov 13, 2024 |
Understanding Membrane Potential Changes
The membrane potential of a neuronal cell is influenced by the permeability of ions, particularly potassium (K+) and sodium (Na+). A shift from –70 mV to –50 mV indicates depolarization, where the inside of the cell becomes less negative.
Factors Causing Shift to –50 mV
- A decrease in K+ permeability:
- K+ ions ty... more
The membrane potential of a neuronal cell is influenced by the permeability of ions, particularly potassium (K+) and sodium (Na+). A shift from –70 mV to –50 mV indicates depolarization, where the inside of the cell becomes less negative.
Factors Causing Shift to –50 mV
- A decrease in K+ permeability:
- K+ ions ty... more
How could we use one compressibility factor chart for all the substances?- a)it would be more convenient
- b)the general shapes of the vapour dome and of the constant temperature lines on the p-v plane can be similar for all substances
- c)their similarity can be exploited using dimensionless properties
- d)all of the mentioned
Correct answer is option 'D'. Can you explain this answer?
How could we use one compressibility factor chart for all the substances?
a)
it would be more convenient
b)
the general shapes of the vapour dome and of the constant temperature lines on the p-v plane can be similar for all substances
c)
their similarity can be exploited using dimensionless properties
d)
all of the mentioned

|
Akash Kulkarni answered • Sep 19, 2024 |
The Convenience of a Single Compressibility Factor Chart
Using one compressibility factor chart for all substances offers several advantages:
1. Greater Convenience
- Consolidating data into a single chart simplifies the analysis process for engineers and scientists.
- It allows users to quickly reference a unified source rather than multiple charts for different ... more
Using one compressibility factor chart for all substances offers several advantages:
1. Greater Convenience
- Consolidating data into a single chart simplifies the analysis process for engineers and scientists.
- It allows users to quickly reference a unified source rather than multiple charts for different ... more
Does hydrolysis of xef6 lead to a redox reaction?

|
Akash Kulkarni answered • Jul 28, 2024 |
Hydrolysis of XeF6 and Redox Reaction
The hydrolysis of XeF6 does lead to a redox reaction. Let's break it down in detail:
Hydrolysis of XeF6:
- XeF6 undergoes hydrolysis in the presence of water to form xenon oxyfluorides and hydrofluoric acid.
- The reaction can be represented as: XeF6 + 3H2O → XeO3 + 6HF
Redox Reaction:
- In this re... more
The hydrolysis of XeF6 does lead to a redox reaction. Let's break it down in detail:
Hydrolysis of XeF6:
- XeF6 undergoes hydrolysis in the presence of water to form xenon oxyfluorides and hydrofluoric acid.
- The reaction can be represented as: XeF6 + 3H2O → XeO3 + 6HF
Redox Reaction:
- In this re... more
Give a brief account on interstitial nitride?

|
Akash Kulkarni answered • Jul 11, 2024 |
Interstitial Nitrides
Interstitial nitrides are a class of compounds that are formed by non-metallic elements occupying the interstitial sites of a metal lattice. These nitrides exhibit unique properties due to the presence of nitrogen atoms within the metal structure.
Formation
- Interstitial nitrides are typically formed by direct ... more
For the metalloprotein hemerythrin the statement that is not true is?

|
Akash Kulkarni answered • Apr 01, 2024 |
Not True Statement about Hemerythrin
Some key points about hemerythrin need to be considered to identify the statement which is not true.
Hemerythrin Overview
- Hemerythrin is a metalloprotein that contains iron and is involved in oxygen transport and storage in certain marine invertebrates.
- It has a unique structure with two iron atoms bound to the prote... more
Some key points about hemerythrin need to be considered to identify the statement which is not true.
Hemerythrin Overview
- Hemerythrin is a metalloprotein that contains iron and is involved in oxygen transport and storage in certain marine invertebrates.
- It has a unique structure with two iron atoms bound to the prote... more
A metal crystallizes in two cubic phases, fcc and bcc whose unit cell lengths are 3.5Å and 3.0Å resp. Calculate the ratio (up to two decimal places) of densities of fcc to bcc.
Correct answer is between '1.25,1.27'. Can you explain this answer?
A metal crystallizes in two cubic phases, fcc and bcc whose unit cell lengths are 3.5Å and 3.0Å resp. Calculate the ratio (up to two decimal places) of densities of fcc to bcc.

|
Akash Kulkarni answered • Apr 01, 2024 |
Explanation:
Densities of fcc and bcc:
- For fcc, the atoms are located at the corners and face centers of the cube. The number of atoms per unit cell in fcc is 4.
- For bcc, the atoms are located at the corners and body center of the cube. The number of atoms per unit cell in bcc is 2.
Calculating the densities:
- The density of a material... more
Densities of fcc and bcc:
- For fcc, the atoms are located at the corners and face centers of the cube. The number of atoms per unit cell in fcc is 4.
- For bcc, the atoms are located at the corners and body center of the cube. The number of atoms per unit cell in bcc is 2.
Calculating the densities:
- The density of a material... more
Which is an example of PN junction diode?- a)Light Emitting Diode
- b)Light dependent resistor
- c)Photo Voltaic cell
- d)Capacitor
Correct answer is option 'A'. Can you explain this answer?
Which is an example of PN junction diode?
a)
Light Emitting Diode
b)
Light dependent resistor
c)
Photo Voltaic cell
d)
Capacitor

|
Akash Kulkarni answered • Feb 21, 2024 |
PN Junction Diode
The correct example of a PN junction diode is a Light Emitting Diode (LED). Let's understand why LED fits the criteria of a PN junction diode:
What is a PN Junction Diode?
A PN junction diode is a semiconductor device that allows the flow of electric current in one direction while blocking it in the opposite direction. It is formed b... more

|
Akash Kulkarni asked • Sep 29, 2023 |
What is the primary advantage of ion-selective electrodes over inert-indicator electrodes in potentiometry?- a)Ion-selective electrodes provide faster results.
- b)Ion-selective electrodes respond to all ions in the solution.
- c)Ion-selective electrodes can selectively respond to specific ions.
- d)Ion-selective electrodes are more durable.
Correct answer is option 'C'. Can you explain this answer?
a)
Ion-selective electrodes provide faster results.
b)
Ion-selective electrodes respond to all ions in the solution.
c)
Ion-selective electrodes can selectively respond to specific ions.
d)
Ion-selective electrodes are more durable.
|
|
Vivek Khatri answered |
The primary advantage of ion-selective electrodes is that they can selectively respond to specific ions in the solution, making them highly selective for particular analytes.

|
Akash Kulkarni asked • Aug 17, 2023 |
Can I access the IIT JAM Chemistry answer key without my application number or registration ID?

|
Shivani Mehta answered |
Accessing IIT JAM Chemistry Answer Key without Application Number or Registration ID
It is not possible to access the IIT JAM Chemistry answer key without your application number or registration ID. The answer key is a confidential document that is made available only to the candidates who have successfully registered for the exam. The application number and registration ID are u... more
It is not possible to access the IIT JAM Chemistry answer key without your application number or registration ID. The answer key is a confidential document that is made available only to the candidates who have successfully registered for the exam. The application number and registration ID are u... more

|
Akash Kulkarni asked • Aug 17, 2023 |
Are there any online study groups or forums where students discuss IIT JAM Chemistry topics and share insights?

|
Shivani Mehta answered |
Yes, there are online study groups and forums where students can discuss IIT JAM Chemistry topics and share insights. These platforms provide a space for students to interact with each other, ask questions, and exchange knowledge related to the subject. One such platform is EduRev, which offers a dedicated section for IIT JAM Chemistry.
Here is a detailed explanation of how EduRev's onl... more
Here is a detailed explanation of how EduRev's onl... more

|
Akash Kulkarni asked • Aug 14, 2023 |
Are there any specific tips to score above the cutoff marks in the IIT JAM Chemistry exam?

|
Sinjini Singh answered |
1. Understand the Exam Pattern and Syllabus:
- Begin by thoroughly understanding the exam pattern and syllabus of the IIT JAM Chemistry exam.
- Take note of the weightage given to different topics and sections.
2. Create a Study Plan:
- Devise a study plan that covers all the important topics and allows you to allocate sufficient time for each subject.
- Include regular revision sessions in your study plan.

|
Akash Kulkarni asked • Aug 02, 2023 |
Can I use rough sheets for calculations during the IIT JAM Chemistry exam?

|
Soumya Sharma answered |
Can I use rough sheets for calculations during the IIT JAM Chemistry exam?
Yes, you are allowed to use rough sheets for calculations during the IIT JAM Chemistry exam. Here are some important details regarding the use of rough sheets:
1. Official Guidelines:
- According to the official guidelines provided by IIT JAM, candidates are allowed to use rough shee... more
Yes, you are allowed to use rough sheets for calculations during the IIT JAM Chemistry exam. Here are some important details regarding the use of rough sheets:
1. Official Guidelines:
- According to the official guidelines provided by IIT JAM, candidates are allowed to use rough shee... more

|
Akash Kulkarni asked • Dec 08, 2022 |
Which type of bond is present between hydrogens in hydrogen molecule?- a)Sigma bond
- b)Pi bond
- c)Ionic bond
- d)Metallic bond
Correct answer is option 'A'. Can you explain this answer?
Which type of bond is present between hydrogens in hydrogen molecule?
a)
Sigma bond
b)
Pi bond
c)
Ionic bond
d)
Metallic bond

|
Tejas Goyal answered |
Explanation:
The type of bond present between the two hydrogen atoms in a hydrogen molecule is a Sigma bond.
Sigma bond:
- A sigma bond is formed when two atomic orbitals overlap head-on, resulting in the sharing of electrons between the two atoms.
- In a hydrogen molecule, each hydrogen atom contributes one electron to form a covalent bond.... more
The type of bond present between the two hydrogen atoms in a hydrogen molecule is a Sigma bond.
Sigma bond:
- A sigma bond is formed when two atomic orbitals overlap head-on, resulting in the sharing of electrons between the two atoms.
- In a hydrogen molecule, each hydrogen atom contributes one electron to form a covalent bond.

|
Akash Kulkarni asked • Dec 04, 2022 |
Which among the following noble gases does not form clathrates?- a)Argon
- b)Xenon
- c)Krypton
- d)Helium
Correct answer is option 'D'. Can you explain this answer?
Which among the following noble gases does not form clathrates?
a)
Argon
b)
Xenon
c)
Krypton
d)
Helium
|
|
Vivek Khatri answered |
Noble gases can form compounds in which the gases are entrapped in the cavities of crystal lattices. Such compounds are called clathrates. Only Argon, Krypton, Xenon and Radon are known to form clathrates among the noble gases.

|
Akash Kulkarni asked • Dec 01, 2022 |
Which of the following statement (s) is/are true about the following eliminations?

(1) Hoffmann product is major product in I (2) Saytzeff product is major product in I (3) Hoffmann product is major product in II (4) Saytzeffproduct is major product in II- a)1 and 2
- b)1 and 4
- c)2 and 3
- d)3 and 4
Correct answer is option 'B'. Can you explain this answer?
Which of the following statement (s) is/are true about the following eliminations?

(1) Hoffmann product is major product in I (2) Saytzeff product is major product in I (3) Hoffmann product is major product in II (4) Saytzeffproduct is major product in II

(1) Hoffmann product is major product in I (2) Saytzeff product is major product in I (3) Hoffmann product is major product in II (4) Saytzeffproduct is major product in II
a)
1 and 2
b)
1 and 4
c)
2 and 3
d)
3 and 4

|
Asf Institute answered |
Here correct is statement 1 and 4 are true. Due to steric hinderence tert-Butyl alcohol anion will attack less substituted C-H bond.
Ethanol anion will attack more substituted C-H bond to form more stable alkene.
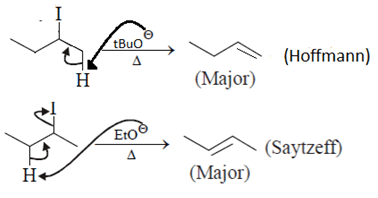
Ethanol anion will attack more substituted C-H bond to form more stable alkene.

Fetching relevant content for you

























