All Exams >
JEE >
Weekly Tests for JEE Preparation >
All Questions
All questions of November Week 3 for JEE Exam
The focal length of a convex lens (refractive index = 1.5) in air is 20 cm. When immersed in water (refractive index = 1.33), its focal length will be- a)20.2cm
- b)78.23 cm
- c)7.23 cm
- d)2.02 cm
Correct answer is option 'B'. Can you explain this answer?
The focal length of a convex lens (refractive index = 1.5) in air is 20 cm. When immersed in water (refractive index = 1.33), its focal length will be
a)
20.2cm
b)
78.23 cm
c)
7.23 cm
d)
2.02 cm
|
|
Nikita Singh answered |
Focal length in air = 20 cm
Refractive index of air-water n₁= 1.33
Refractive index of air - glass n₂= 1.5
For focal length in air,
Using formula of lens
1/fair={(n2/n1)-1}(1/R1)-(1/R2)
Put the value into the formula
1/20={(1.5/1)-1}{(1/R1)-(1/R2)}
1/20=0.5{(1/R1)-(1/R2)}…1
We need to calculate the focal length in water
Using formula of lens
1/fwater={(1.5/1.33)-1}{(1/R1)-(1/R2)}
1/fwater=0.128{(1/R1)-(1/R2)}….2
fwater/20=0.5/0.128
fwater=78.125cm
Refractive index of air-water n₁= 1.33
Refractive index of air - glass n₂= 1.5
For focal length in air,
Using formula of lens
1/fair={(n2/n1)-1}(1/R1)-(1/R2)
Put the value into the formula
1/20={(1.5/1)-1}{(1/R1)-(1/R2)}
1/20=0.5{(1/R1)-(1/R2)}…1
We need to calculate the focal length in water
Using formula of lens
1/fwater={(1.5/1.33)-1}{(1/R1)-(1/R2)}
1/fwater=0.128{(1/R1)-(1/R2)}….2
fwater/20=0.5/0.128
fwater=78.125cm
Power of the lens is -40, its focal length is - a)4m
- b)-40m
- c)-0.25m
- d)-25m
Correct answer is option 'C'. Can you explain this answer?
Power of the lens is -40, its focal length is
a)
4m
b)
-40m
c)
-0.25m
d)
-25m
|
|
Jyoti Sengupta answered |
The power of a lens is the reciprocal of the focal length with measurement in metres. The unit is diopter.
It is given that the focal length of a convex lens is 10 cm = 0.1 m.
⇒ The power of the lens is 1/0.1= 10 diopter.
A convex and a concave mirror of radii 10 cm each are facing each other and 15 cm apart. A point object is placed midway between them. Then position of the final image if the reflection first takes place at the concave mirror and then in the convex mirror is- a)at the pole of the concave mirror
- b)at the pole of the convex mirror
- c)5 cm behind the convex mirror
- d)coincident with the object itself
Correct answer is option 'B'. Can you explain this answer?
A convex and a concave mirror of radii 10 cm each are facing each other and 15 cm apart. A point object is placed midway between them. Then position of the final image if the reflection first takes place at the concave mirror and then in the convex mirror is
a)
at the pole of the concave mirror
b)
at the pole of the convex mirror
c)
5 cm behind the convex mirror
d)
coincident with the object itself

|
Aayush Agarwal answered |
It will be best understood if you make a scale diagram. I will advice you do so and simultaneously read the answer. at first the reflection takes place at concave mirror. the objeCt is at a distance of 7.5cm from it. and 2.5cm from its focus. thereby the image shall be made in front of the mirror. next the image made will act as an object for the convex Mirror. and then it will make the image at its pole. if you arent still able to understand, do let me know
______ mirror is called as diverging mirror- a)Concave
- b)Plane
- c)Convex
- d)Both b and c
Correct answer is option 'C'. Can you explain this answer?
______ mirror is called as diverging mirror
a)
Concave
b)
Plane
c)
Convex
d)
Both b and c
|
|
Preeti Iyer answered |
Concave mirror is called a converging mirror because parallel rays of light fall on the mirror they converge at a point called focus. Convex mirror is called a diverging mirror because parallel rays of light fall on it they diverge after reflection.
In a concave mirror when the object is located beyond C the magnification is- a)More than 1
- b)Equal to 1
- c)Less than 1
- d)Both a and b
Correct answer is option 'C'. Can you explain this answer?
In a concave mirror when the object is located beyond C the magnification is
a)
More than 1
b)
Equal to 1
c)
Less than 1
d)
Both a and b
|
|
Rajeev Saxena answered |
In the animation above, a right-side-up object is located above the principal axis at a position beyond the center of curvature (C). The ray diagram shows that the image of this object is located as an upside-down image positioned between the center of curvature (C) and the focal point (F). In fact, it can be generalized that anytime the object is located beyond the center of curvature, the image will be located somewhere between the center of curvature and the focal point. In such cases, the image will be inverted and reduced in size (i.e., smaller than the object). Such images are called real images because they are formed by the actual convergence of reflected light rays at the image location. Real images are always formed on the same side of the mirror as the object.
Newtonian reflecting type telescope uses- a)Concave mirror
- b)Convex lens
- c)Concave lens
- d)Convex mirror
Correct answer is option 'B'. Can you explain this answer?
Newtonian reflecting type telescope uses
a)
Concave mirror
b)
Convex lens
c)
Concave lens
d)
Convex mirror
|
|
Ishan Choudhury answered |
A reflecting telescope (also called a reflector) is a telescope that uses a single or a combination of curved mirrors that reflect light and form an image. The reflecting telescope was invented in the 17th century, by Isaac Newton, as an alternative to the refracting telescope which, at that time, was a design that suffered from severe chromatic aberration. Although reflecting telescopes produce other types of optical aberrations, it is a design that allows for very large diameter objectives.
Astronomical (reflecting) telescopes. In a reflecting telescope, instead of a convex objective lens, a concave mirror is used to collect parallel rays from the object and form an image at the focal point. Then the convex eyepiece lens is used to magnify this image for the viewer.
An image is upright and reduced in size. Which mirror is used to form such an image?- a)Convex
- b)Concave
- c)Plane
- d)Both a and c
Correct answer is option 'D'. Can you explain this answer?
An image is upright and reduced in size. Which mirror is used to form such an image?
a)
Convex
b)
Concave
c)
Plane
d)
Both a and c
|
|
Nikita Singh answered |
Only Convex Mirror can form an image which is upright and reduced in size.
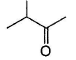
In which of the following compounds, enol form exist? - a)C6H5COCH3
- b)C6H5CHO
- c)

- d)Both (a) and (c)
Correct answer is option 'D'. Can you explain this answer?
In which of the following compounds, enol form exist?
a)
C6H5COCH3
b)
C6H5CHO
c)
d)
Both (a) and (c)
|
|
Preeti Khanna answered |
Both option (a) and option (c) forms enol but option (b) does not form enol.
Which can be deduced correctly regarding keto-enol tautomerism in general?- a)Increasing temperature increases the enol content at equilibrium
- b)Mono-enols are usually more stable than dienols
- c)Enols of ketones are generally more stable than enols of aliphatic aldehydes
- d)Keto-enol taytomerism is catalysed by both acidic and basic catalys
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Which can be deduced correctly regarding keto-enol tautomerism in general?
a)
Increasing temperature increases the enol content at equilibrium
b)
Mono-enols are usually more stable than dienols
c)
Enols of ketones are generally more stable than enols of aliphatic aldehydes
d)
Keto-enol taytomerism is catalysed by both acidic and basic catalys

|
Ishita Deshpande answered |
Increasing temperature increases equilibrium content of less stable enol tautomers.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
Only One Option Correct TypeDirection (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. In hexane-2,4-dione, how many different mono-enols are possible?- a)2
- b)3
- c)4
- d)7
Correct answer is option 'D'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
In hexane-2,4-dione, how many different mono-enols are possible?
a)
2
b)
3
c)
4
d)
7
|
|
Devanshi Mehta answered |
Possible Mono-enols in Hexane-2,4-dione
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
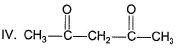
What is the correct order of equilibrium enol content of the following compounds?I. CH3COCH3
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH- a)I > II > III > IV
- b)I > IV > III > II
- c)IV > II > III > I
- d)III > IV > II > I
Correct answer is option 'D'. Can you explain this answer?
What is the correct order of equilibrium enol content of the following compounds?
I. CH3COCH3
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
a)
I > II > III > IV
b)
I > IV > III > II
c)
IV > II > III > I
d)
III > IV > II > I

|
Asha Nair answered |
A 1,3-diketo compound forms more stable enol than a monocarbonyls. Also ester group forms less stable enol than carbonyls. Hence, III, a 1 , 3-diketo ne form s highest enol content while I (monocarbonyl) forms least enol content at equilibrium.
The correct statement(s) regarding hydrates of aldehyde and ketone is/are- a)Usually hydrates have lower thermodynamic stability than anhydrous form
- b)Hydrate content of acetone is greater in water than in hexane
- c)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18
- d)C6H5CHO has greater hydrate content than p-nitrobenzaldehyd
Correct answer is option 'A,B,C'. Can you explain this answer?
The correct statement(s) regarding hydrates of aldehyde and ketone is/are
a)
Usually hydrates have lower thermodynamic stability than anhydrous form
b)
Hydrate content of acetone is greater in water than in hexane
c)
CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18
d)
C6H5CHO has greater hydrate content than p-nitrobenzaldehyd
|
|
Nisha Kulkarni answered |
Hydrates of aldehydes and ketones are less stable than anhydrous form (gem diols are unstable).
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
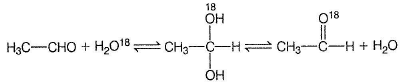
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
If butanone is treated with D2O18/DCI, isotopic exchange occur. What maximum gain in molecular mass is possible in the present case?
Correct answer is '7'. Can you explain this answer?
If butanone is treated with D2O18/DCI, isotopic exchange occur. What maximum gain in molecular mass is possible in the present case?

|
Asha Nair answered |
Gain of 7 units in molar mass is observed, five units due to 'D' and tw o units due to `O18'.
In mirrors how can we differentiate real image from the virtual image- a)Real image is always inverted whereas virtual image is erect.
- b)Real image is always twice in size as compared to virtual.
- c)Real and virtual both are same.
- d)Virtual image is always inverted whereas real is erect.
Correct answer is option 'A'. Can you explain this answer?
In mirrors how can we differentiate real image from the virtual image
a)
Real image is always inverted whereas virtual image is erect.
b)
Real image is always twice in size as compared to virtual.
c)
Real and virtual both are same.
d)
Virtual image is always inverted whereas real is erect.

|
Akshay Shah answered |
1 When the incident rays arise from a given object, then it is known as a real object.
2 Concave mirror or a converging lens are used to produce a real inverted image, wherein the object should be located in front of the lens or mirror, at a place farther than the focus.
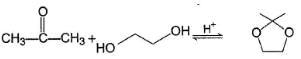
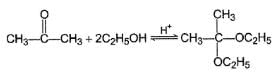
What is the major product in the following reaction?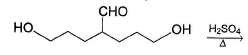
- a)
- b)
- c)
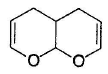
- d)
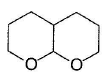
Correct answer is option 'D'. Can you explain this answer?
What is the major product in the following reaction?
a)
b)
c)
d)

|
Pragati Choudhury answered |
Acetal is form ed by cyclisation
Comprehension Type Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageIn general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.Q. The major final product in the following reaction is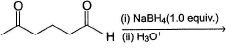
- a)

- b)

- c)
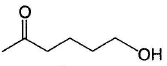
- d)Mixture of (b) and (c)
Correct answer is option 'C'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Q.
The major final product in the following reaction is
a)
b)
c)
d)
Mixture of (b) and (c)

|
Ameya Tiwari answered |
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.Consider the following reaction,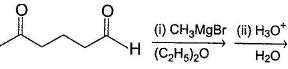 Q. Major product is
Q. Major product is - a)
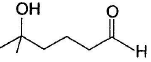
- b)

- c)
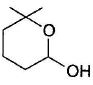
- d)
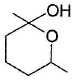
Correct answer is option 'D'. Can you explain this answer?
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Consider the following reaction,
Q.
Major product is
a)
b)
c)
d)

|
Sankar Chakraborty answered |
One or More than One Options Correct TypeDirection (Q. Nos. 15-19) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q.Which of the following has (have) more than one enol tautomers?- a)
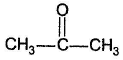
- b)
- c)CH3CH2CHO
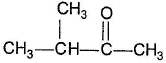
- d)
Correct answer is option 'B,C'. Can you explain this answer?
One or More than One Options Correct Type
Direction (Q. Nos. 15-19) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Which of the following has (have) more than one enol tautomers?
a)
b)
c)
CH3CH2CHO
d)

|
Sai Chakraborty answered |
Arrange the following in the increasing order of stability of their most stable enol.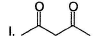
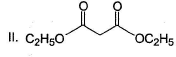
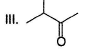
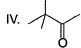
- a) I < II < III < IV
- b)IV < III < II < I
- c)II < I < IV < III
- d)III < IV < II < I
Correct answer is option 'B'. Can you explain this answer?
Arrange the following in the increasing order of stability of their most stable enol.
a)
I < II < III < IV
b)
IV < III < II < I
c)
II < I < IV < III
d)
III < IV < II < I

|
Sai Chakraborty answered |
Diketo (I) forms highest enol content due to stabilisation of enol by intermolecular H-bonding. Electron donating resonance effect by ester group slightly decreases enol content.
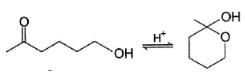
For which of the following equilibrium, Kc is greater than 1?- a)
- b)
- c)
- d)
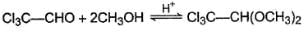
Correct answer is option 'A,B'. Can you explain this answer?
For which of the following equilibrium, Kc is greater than 1?
a)

b)

c)

d)
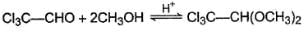

|
Kavya Das answered |
Five and six-membered cyclic hemiacetals are more stable than its hydroxy aldehydes/ketones, predominate at equilibrium. Cyclic acetals are highly stable, predominate at equilibrium.
Arrange the following in the increasing order of hydrate content at equilibrium in aqueous solutionI. H2CO
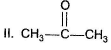
III. C6H5CHO

- a)I < II < III < IV
- b)IV < III < II < I
- c)III < IV < II < I
- d)Ill < IV < I < II
Correct answer is option 'C'. Can you explain this answer?
Arrange the following in the increasing order of hydrate content at equilibrium in aqueous solution
I. H2CO
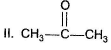
III. C6H5CHO

III. C6H5CHO
a)
I < II < III < IV
b)
IV < III < II < I
c)
III < IV < II < I
d)
Ill < IV < I < II
|
|
Shalini Basu answered |
Due to negligible steric hindrance, form aldehyde form s large hydrate content at equilibrium. Also electron withdrawing group in (IV) increases hydrate content compared to III.
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.Q. How the following transformation can be best brought about?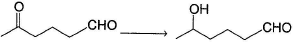
- a){(CH3)2CHO}3AI;H3O+
- b)
 then NaBH4 followed by H3O+
then NaBH4 followed by H3O+ - c)H2/Ni; high p and T
- d)N2H4/NaOH/Heat
Correct answer is option 'B'. Can you explain this answer?
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Q.
How the following transformation can be best brought about?
a)
{(CH3)2CHO}3AI;H3O+
b)
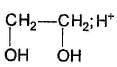
then NaBH4 followed by H3O+
c)
H2/Ni; high p and T
d)
N2H4/NaOH/Heat

|
Pragati Choudhury answered |
Aldehyde being more reactive, protected first by acetal formation followed by reduction of ketone group.
Arrange the following in the increasing order of acidic strength I. H2CO
II. CH3CHO
III. C6H5CH2CHO
- a)I < II < III < IV
- b)IV < III < II < I
- c)III < IV < II < I
- d)III < IV < I < II
Correct answer is option 'A'. Can you explain this answer?
Arrange the following in the increasing order of acidic strength
I. H2CO
II. CH3CHO
III. C6H5CH2CHO

II. CH3CHO
III. C6H5CH2CHO
a)
I < II < III < IV
b)
IV < III < II < I
c)
III < IV < II < I
d)
III < IV < I < II

|
Maitri Sharma answered |
IV is most acidic as its conjugate base is resonance stabilised by two carbonyl groups, followed by III whose conjugate base is resonance stabilised by a carbonyl group and phenyi ring.
For which of the following equilibrium, Kc < 1 ?- a)
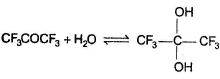
- b)
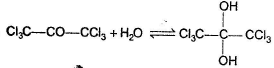
- c)
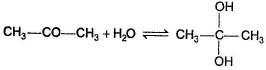
- d)
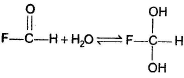
Correct answer is option 'C'. Can you explain this answer?
For which of the following equilibrium, Kc < 1 ?
a)
b)
c)
d)

|
Nidhi Nambiar answered |
In the absence of any special stability factor, a hydrate of ketone is highly unstable.
Consider the following reaction,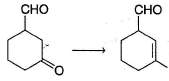 Q. The appropriatensequence of reagent that can best bring about the above conversion is
Q. The appropriatensequence of reagent that can best bring about the above conversion is- a)(i) CH3MgBr (1.0 equiv.) (ii) Conc. H2SO4/ Heat
- b)(i) CH3Li(1.0 equiv.)(ii)Conc. H2SO4/Heat
- c)(i) C2H5OH (2.0 equiv.); H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat
- d)(i) HOCH2CH2OH; H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat (iv) H3O+/H2O
Correct answer is option 'D'. Can you explain this answer?
Consider the following reaction,
Q.
The appropriatensequence of reagent that can best bring about the above conversion is
a)
(i) CH3MgBr (1.0 equiv.) (ii) Conc. H2SO4/ Heat
b)
(i) CH3Li(1.0 equiv.)(ii)Conc. H2SO4/Heat
c)
(i) C2H5OH (2.0 equiv.); H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat
d)
(i) HOCH2CH2OH; H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat (iv) H3O+/H2O

|
Dishani Kulkarni answered |
Which of the following observation does not establish the existence of keto-enol tautomerism in acetone?- a)When treated with DCI in D2O, hexadeuterated acetone is recovered
- b)When treated with D2O/NaOD, hexadeuterated acetone is recovered
- c)When treated with dil. H2SO4 in H2O18,
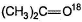 is obtained
is obtained - d)All of the above establishes keto-enol tautomerism
Correct answer is option 'C'. Can you explain this answer?
Which of the following observation does not establish the existence of keto-enol tautomerism in acetone?
a)
When treated with DCI in D2O, hexadeuterated acetone is recovered
b)
When treated with D2O/NaOD, hexadeuterated acetone is recovered
c)
When treated with dil. H2SO4 in H2O18, 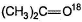 is obtained
is obtained
d)
All of the above establishes keto-enol tautomerism

|
Pragati Choudhury answered |
The above exchange occur due to existence of hydrate equilibrium.
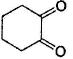
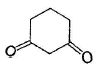
Among the following compounds, one that will not show keto-enol tautomerism is- a)

- b)
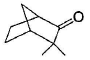
- c)
- d)
Correct answer is option 'B'. Can you explain this answer?
Among the following compounds, one that will not show keto-enol tautomerism is
a)
b)
c)
d)

|
Kavya Das answered |
sp2 hybridisation is very less stable at bridgehead carbon of a bicyclic compound.
Hemiacetals are usually unstable whereas acetals are stable. Which has the most stable hemiacetal?- a)
- b)
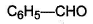
- c)

- d)
Correct answer is option 'D'. Can you explain this answer?
Hemiacetals are usually unstable whereas acetals are stable. Which has the most stable hemiacetal?
a)
b)
c)
d)

|
Kavya Das answered |
If a hemiacetal is five or six membered cyclic, they are stable.
Consider the reaction below,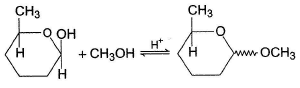 The correct statement(s) regarding the above process is/are
The correct statement(s) regarding the above process is/are- a)Equilibrium favours product
- b)Pair of enantiomers of acetals are formed
- c)Pair of diastereomers of acetals are formed
- d)Equilibrium favours reactant
Correct answer is option 'A,C'. Can you explain this answer?
Consider the reaction below,
The correct statement(s) regarding the above process is/are
a)
Equilibrium favours product
b)
Pair of enantiomers of acetals are formed
c)
Pair of diastereomers of acetals are formed
d)
Equilibrium favours reactant

|
Ameya Tiwari answered |
Product is an acetal, more stable than hemiacetal reactant. Acetal is formed via carbocation, both diastereomers are formed.
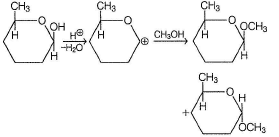
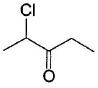
Which of the following has greater enol content than keto counter part?- a)C6H5—CH2CHO
- b)
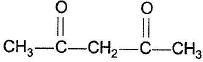
- c)
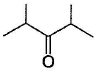
- d)
Correct answer is option 'B'. Can you explain this answer?
Which of the following has greater enol content than keto counter part?
a)
C6H5—CH2CHO
b)
c)
d)

|
Pragati Choudhury answered |
It is 1, 3-dik eto compound, forms stable enol.
Chapter doubts & questions for November Week 3 - Weekly Tests for JEE Preparation 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of November Week 3 - Weekly Tests for JEE Preparation in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Related JEE Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup










