All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of December Week 3 for NEET Exam
Each cardiac cycle takes 0.8 seconds to occur. Calculate how many cardiac cycles occur in 4 minutes ?- a)250
- b)75
- c)400
- d)300
Correct answer is option 'D'. Can you explain this answer?
Each cardiac cycle takes 0.8 seconds to occur. Calculate how many cardiac cycles occur in 4 minutes ?
a)
250
b)
75
c)
400
d)
300

|
Nilotpal Gupta answered |
4 min = 240 sec. Therefore, 240/0.8 = 300 cardiac cycles.
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?- a)Room temperature would decrease
- b)Room temperature would increase
- c)Room temperature would be same as the temperature inside the refrigerator
- d)Room temperature would not be effected
Correct answer is option 'B'. Can you explain this answer?
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?
a)
Room temperature would decrease
b)
Room temperature would increase
c)
Room temperature would be same as the temperature inside the refrigerator
d)
Room temperature would not be effected
|
|
Riya Banerjee answered |
If you leave the door open, heat is merely recycled from the room into therefrigerator, then back into the room. A net room temperature increase wouldresult from the heat of the motor that would be constantly running to move energy around in a circle.
Manoj has AB blood group, so he will have the following antibodies in his blood plasma :- a)a antibody
- b)both a and b antibodies
- c)b antibody
- d)No antibodies are present
Correct answer is option 'D'. Can you explain this answer?
Manoj has AB blood group, so he will have the following antibodies in his blood plasma :
a)
a antibody
b)
both a and b antibodies
c)
b antibody
d)
No antibodies are present

|
Kunal Rane answered |
AB blood group does not have any antibodies in plasma.
Blood plasma proteins :
i. decrease in their level causes excessive absorption of water from tissues into blood.
ii. they maintain osmotic pressure.
- a)both are correct.
- b)both are wrong
- c)Statement i) is wrong and ii) is correct.
- d)Statement i) is correct and ii) is wrong
Correct answer is option 'C'. Can you explain this answer?
Blood plasma proteins :
i. decrease in their level causes excessive absorption of water from tissues into blood.
ii. they maintain osmotic pressure.
i. decrease in their level causes excessive absorption of water from tissues into blood.
ii. they maintain osmotic pressure.
a)
both are correct.
b)
both are wrong
c)
Statement i) is wrong and ii) is correct.
d)
Statement i) is correct and ii) is wrong

|
Ishaan Menon answered |
Let's analyze the statements about blood plasma proteins:
Statement i: "Decrease in their level causes excessive absorption of water from tissues into blood."
- This statement is incorrect. A decrease in the level of blood plasma proteins would actually result in a decrease in osmotic pressure, leading to less water being drawn from tissues into the blood. Instead, it would cause water to accumulate in the tissues, leading to edema.
Statement ii: "They maintain osmotic pressure."
- This statement is correct. Blood plasma proteins, especially albumin, play a crucial role in maintaining the osmotic pressure of the blood.
Therefore, the correct option is:
3. Statement i) is wrong and ii) is correct.
Blood pressure is expressed as the ratio of systolic over diastolic pressure. The difference between systolic and diastolic pressure is called pulse pressure. What will be its value for a normal healthy adult?- a)40 mm Hg
- b)30 mm Hg
- c)70 mm Hg
- d)50 mm Hg
Correct answer is option 'A'. Can you explain this answer?
Blood pressure is expressed as the ratio of systolic over diastolic pressure. The difference between systolic and diastolic pressure is called pulse pressure. What will be its value for a normal healthy adult?
a)
40 mm Hg
b)
30 mm Hg
c)
70 mm Hg
d)
50 mm Hg

|
Tejas Chavan answered |
Blood pressure is represented as the ratio of systolic over diastolic pressure. The difference between systolic and diastolic pressure is called pulse pressure. The value of normal healthy pulse pressure is 40 mm Hg.
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?- a)20,2
- b)7.9,6.5
- c)9.8,7.3
- d)8.9,7.3
Correct answer is option 'D'. Can you explain this answer?
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?
a)
20,2
b)
7.9,6.5
c)
9.8,7.3
d)
8.9,7.3
|
|
Neha Joshi answered |
We know that the efficiency of refrigeration for a refrigerator is T2 / T1 + T2
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
The second law of thermodynamics says- a)Coefficient of performance can never be infinite for refrigerator
- b)Heat released to the cold reservoir can be zero
- c)Ideal gas can expand infinitely
- d)Efficiency of a heat engine can be 100%.
Correct answer is option 'A'. Can you explain this answer?
The second law of thermodynamics says
a)
Coefficient of performance can never be infinite for refrigerator
b)
Heat released to the cold reservoir can be zero
c)
Ideal gas can expand infinitely
d)
Efficiency of a heat engine can be 100%.
|
|
Raghav Bansal answered |
The second law of thermodynamics gives a fundamental limitation to the efficiency of a heat engine and the coefficient of performance of a refrigerator. It says that the efficiency of a heat engine can never be unity or 100%, this implies that the heat released to the cold reservoir can never be made zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
Which word is defined by this statement: A measure of this disorder, or randomness?- a)energy
- b)enthalpy
- c)mass
- d)entropy
Correct answer is option 'D'. Can you explain this answer?
Which word is defined by this statement: A measure of this disorder, or randomness?
a)
energy
b)
enthalpy
c)
mass
d)
entropy
|
|
Janhavi Rane answered |
Understanding Entropy
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____- a)as close to unity, be different
- b)as close to unity, match
- c)as close to zero, match
- d)as close to zero, be different
Correct answer is option 'B'. Can you explain this answer?
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____
a)
as close to unity, be different
b)
as close to unity, match
c)
as close to zero, match
d)
as close to zero, be different
|
|
Raghav Bansal answered |
If first law efficiency is close to unity, the all the energy carried in by heat transfer is used and no heat is lost to the surroundings.
The mass of tissue seen in the left corner of the right atrium close to the atri-ventricular septum is- a)Purkinje fibres
- b)Bundle of His
- c)AVN
- d)SAN
Correct answer is option 'C'. Can you explain this answer?
The mass of tissue seen in the left corner of the right atrium close to the atri-ventricular septum is
a)
Purkinje fibres
b)
Bundle of His
c)
AVN
d)
SAN

|
Krish Chakraborty answered |
The mass of tissue seen in the lower left corner of the right atrium close to the atrio-ventricular septum called the atrio-ventricular node (AVN).
When blood clot starts contracting, a pale yellow fluid starts oozing out. Its name and composition is :- a)Serum = plasma – [thrombin + blood corpuscles]
- b)Serum = plasma – [fibrinogen + blood corpuscles]
- c)Sera = blood corpuscles – [thrombin + fibrin]
- d)Serum = dissolved fibrin – [plasma + blood corpuscles]
Correct answer is option 'B'. Can you explain this answer?
When blood clot starts contracting, a pale yellow fluid starts oozing out. Its name and composition is :
a)
Serum = plasma – [thrombin + blood corpuscles]
b)
Serum = plasma – [fibrinogen + blood corpuscles]
c)
Sera = blood corpuscles – [thrombin + fibrin]
d)
Serum = dissolved fibrin – [plasma + blood corpuscles]

|
Arnav Iyer answered |
Plasma without the clotting factors is called serum. The clotting factors include fibrinogen and blood corpuscles
In the human heart :
i. The valve between right atrium and right ventricle is tricuspid while valve between left atrium and left ventricle is mitral valve.
ii. Openings of the right and the left ventricles into the pulmonary artery and the aorta are guarded by semilunar valves.
iii. ‘Lub’ the first sound which is low pitched is caused by the closure of semilunar valves while ‘Dub’ the second sound which is high pitched is caused by the closure of bicuspid and tricuspid valves.- a)only ii) and iii) are correct
- b)only i) and ii) are correct
- c)only iii) is correct
- d)only ii) is correct
Correct answer is option 'B'. Can you explain this answer?
In the human heart :
i. The valve between right atrium and right ventricle is tricuspid while valve between left atrium and left ventricle is mitral valve.
ii. Openings of the right and the left ventricles into the pulmonary artery and the aorta are guarded by semilunar valves.
iii. ‘Lub’ the first sound which is low pitched is caused by the closure of semilunar valves while ‘Dub’ the second sound which is high pitched is caused by the closure of bicuspid and tricuspid valves.
i. The valve between right atrium and right ventricle is tricuspid while valve between left atrium and left ventricle is mitral valve.
ii. Openings of the right and the left ventricles into the pulmonary artery and the aorta are guarded by semilunar valves.
iii. ‘Lub’ the first sound which is low pitched is caused by the closure of semilunar valves while ‘Dub’ the second sound which is high pitched is caused by the closure of bicuspid and tricuspid valves.
a)
only ii) and iii) are correct
b)
only i) and ii) are correct
c)
only iii) is correct
d)
only ii) is correct

|
Rajeev Sharma answered |
The first heart sound (lub) is associated with the closure of the tricuspid and bicuspid valves whereas the second heart sound (dub) is associated with the closure of the semilunar valves.
Meghna suffers from allergic asthma. After a blood test her leucocyte count displayed an abnormal increase in the number of :- a)Eosinophils
- b)Monocytes
- c)Neutrophils
- d)Basophils
Correct answer is option 'A'. Can you explain this answer?
Meghna suffers from allergic asthma. After a blood test her leucocyte count displayed an abnormal increase in the number of :
a)
Eosinophils
b)
Monocytes
c)
Neutrophils
d)
Basophils

|
Soumya Ahuja answered |
In case of allergic asthma, leucocyte count of blood test shows abnormal increase in the number of Eosinophils
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process- a)Zeroth law of thermodynamics
- b)Third law of thermodynamics
- c)First law of thermodynamics
- d)Second law of thermodynamics
Correct answer is option 'D'. Can you explain this answer?
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process
a)
Zeroth law of thermodynamics
b)
Third law of thermodynamics
c)
First law of thermodynamics
d)
Second law of thermodynamics
|
|
Rajat Patel answered |
Refrigerator follows the principle of clausius statement of second law of thermodynamics. It does not violate second law of thermodynamics because it takes energy to transfer heat from low temperature body to high temperature body. Electrical work is given to refrigerator to extract heat from low temperature body and to transfer it to higher temperature body. If any refrigerator is transferring heat from low temperature body to higher temperature body without any external energy then we can say that it violates second law of thermodynamics.But in actual it takes energy to do.
Which of the following is an example of heat pump?- a)Internal combustion engine
- b)Blower heater
- c)Refrigerator
- d)Carnot engine
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of heat pump?
a)
Internal combustion engine
b)
Blower heater
c)
Refrigerator
d)
Carnot engine
|
|
Om Desai answered |
A heat pump is an electrical device that heats a building by pumping heat in from the cold outside. In other words, it’s the same as a refrigerator, but its purpose is to warm the hot reservoir rather than to cool the cold reservoir (even though it does both).
Lymph will transport :- a)digested carbohydrates
- b)oxyhemoglobin
- c)digested fats
- d)digested proteins
Correct answer is option 'C'. Can you explain this answer?
Lymph will transport :
a)
digested carbohydrates
b)
oxyhemoglobin
c)
digested fats
d)
digested proteins

|
Bhargavi Choudhury answered |
Fats are absorbed through lymph in the lacteals present in the intestinal villi.
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N- a)(i) sp2, trigonal planar; (ii) sp3, tetrahedral
- b)(i) sp3, tetrahedral; (ii) sp, linear
- c)(i) sp, linear; (ii) sp2, trigonal planar
- d)(i) sp2, trigonal planar, (ii) sp2, trigonal planar
Correct answer is option 'B'. Can you explain this answer?
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N
(i) CH3F
(ii) HC ≡ N
a)
(i) sp2, trigonal planar; (ii) sp3, tetrahedral
b)
(i) sp3, tetrahedral; (ii) sp, linear
c)
(i) sp, linear; (ii) sp2, trigonal planar
d)
(i) sp2, trigonal planar, (ii) sp2, trigonal planar
|
|
Rajesh Gupta answered |
CH3F - sp3 hybridised carbon, tetrahedral shape
(ii) HC = N - sp hybridised carbon, linear shape.
(ii) HC = N - sp hybridised carbon, linear shape.
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N- a)(i) sp2, trigonal planar; (ii) sp3, tetrahedral
- b)(i) sp3, tetrahedral; (ii) sp, linear
- c)(i) sp, linear; (ii) sp2, trigonal planar
- d)(i) sp2, trigonal planar, (ii) sp2, trigonal planar
Correct answer is option 'B'. Can you explain this answer?
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N
(i) CH3F
(ii) HC ≡ N
a)
(i) sp2, trigonal planar; (ii) sp3, tetrahedral
b)
(i) sp3, tetrahedral; (ii) sp, linear
c)
(i) sp, linear; (ii) sp2, trigonal planar
d)
(i) sp2, trigonal planar, (ii) sp2, trigonal planar
|
|
Pankaj Dasgupta answered |
(i) CH3F:
The central atom in CH3F is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms four covalent bonds in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to each hydrogen atom, one single bond to the fluorine atom, and one lone pair on carbon. This gives us a total of four regions of electron density.
The hybridization of an atom with four regions of electron density is sp3. Therefore, the carbon atom in CH3F is sp3 hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, the lone pair and the three bond pairs are arranged in a tetrahedral geometry. However, the presence of a lone pair causes a distortion in the shape.
The lone pair occupies more space than the bond pairs and exerts a stronger repulsion. As a result, the bond angle between the three hydrogen atoms and the fluorine atom is slightly less than 109.5 degrees.
Therefore, the shape of CH3F is trigonal pyramidal.
(ii) HC:
The central atom in HC is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms one covalent bond in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to the hydrogen atom. This gives us a total of one region of electron density.
The hybridization of an atom with one region of electron density is sp. Therefore, the carbon atom in HC is sp hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, there is only one bond pair, so there is no specific shape associated with HC. However, the bond angle between the carbon and hydrogen atoms is approximately 180 degrees.
Therefore, the shape of HC is linear.
The central atom in CH3F is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms four covalent bonds in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to each hydrogen atom, one single bond to the fluorine atom, and one lone pair on carbon. This gives us a total of four regions of electron density.
The hybridization of an atom with four regions of electron density is sp3. Therefore, the carbon atom in CH3F is sp3 hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, the lone pair and the three bond pairs are arranged in a tetrahedral geometry. However, the presence of a lone pair causes a distortion in the shape.
The lone pair occupies more space than the bond pairs and exerts a stronger repulsion. As a result, the bond angle between the three hydrogen atoms and the fluorine atom is slightly less than 109.5 degrees.
Therefore, the shape of CH3F is trigonal pyramidal.
(ii) HC:
The central atom in HC is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms one covalent bond in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to the hydrogen atom. This gives us a total of one region of electron density.
The hybridization of an atom with one region of electron density is sp. Therefore, the carbon atom in HC is sp hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, there is only one bond pair, so there is no specific shape associated with HC. However, the bond angle between the carbon and hydrogen atoms is approximately 180 degrees.
Therefore, the shape of HC is linear.
Kelvin- Planck statement states that- a)The process whose sole result is transfer of heat from a colder object to a hotter object is not possible
- b)Irreversible processes can be made reversible under certain conditions
- c)No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work
- d)Heat flows from colder body to hotter body
Correct answer is option 'C'. Can you explain this answer?
Kelvin- Planck statement states that
a)
The process whose sole result is transfer of heat from a colder object to a hotter object is not possible
b)
Irreversible processes can be made reversible under certain conditions
c)
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work
d)
Heat flows from colder body to hotter body
|
|
Jyoti Kumar answered |
The Kelvin-Planck statement is a fundamental principle of thermodynamics that is applicable to all heat engines. It states that:
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work.
This statement implies that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. In other words, it is impossible to have a 100% efficient heat engine.
Explanation:
To understand the Kelvin-Planck statement, we need to have a basic understanding of heat engines. A heat engine is a device that converts heat into work. It operates on the principle of the Carnot cycle, which involves four processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. The efficiency of a heat engine is defined as the ratio of the work output to the heat input. According to the second law of thermodynamics, the efficiency of a heat engine cannot exceed the efficiency of a reversible heat engine operating between the same two reservoirs.
The Kelvin-Planck statement is based on the fact that any heat engine must reject some heat to a low-temperature reservoir in order to operate. This means that not all of the heat energy can be converted into useful work. The statement implies that there must always be some waste heat that cannot be utilized to produce work. This is because all natural processes tend to move towards a state of maximum entropy, and the conversion of heat into work is a process that results in a decrease in entropy. Therefore, it is impossible to have a heat engine that can convert all of the heat energy it absorbs into useful work.
Conclusion:
In conclusion, the Kelvin-Planck statement is a fundamental principle of thermodynamics that states that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. This statement is based on the second law of thermodynamics, which states that all natural processes tend to move towards a state of maximum entropy. The Kelvin-Planck statement has important implications for the design and operation of heat engines, and it sets a fundamental limit on the efficiency of such devices.
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work.
This statement implies that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. In other words, it is impossible to have a 100% efficient heat engine.
Explanation:
To understand the Kelvin-Planck statement, we need to have a basic understanding of heat engines. A heat engine is a device that converts heat into work. It operates on the principle of the Carnot cycle, which involves four processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. The efficiency of a heat engine is defined as the ratio of the work output to the heat input. According to the second law of thermodynamics, the efficiency of a heat engine cannot exceed the efficiency of a reversible heat engine operating between the same two reservoirs.
The Kelvin-Planck statement is based on the fact that any heat engine must reject some heat to a low-temperature reservoir in order to operate. This means that not all of the heat energy can be converted into useful work. The statement implies that there must always be some waste heat that cannot be utilized to produce work. This is because all natural processes tend to move towards a state of maximum entropy, and the conversion of heat into work is a process that results in a decrease in entropy. Therefore, it is impossible to have a heat engine that can convert all of the heat energy it absorbs into useful work.
Conclusion:
In conclusion, the Kelvin-Planck statement is a fundamental principle of thermodynamics that states that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. This statement is based on the second law of thermodynamics, which states that all natural processes tend to move towards a state of maximum entropy. The Kelvin-Planck statement has important implications for the design and operation of heat engines, and it sets a fundamental limit on the efficiency of such devices.
Which type of hybridisation of each carbon is there in the compound?
CH3 - CH = CH - CN- a)sp3, sp2, sp2, sp
- b)sp3, sp2, sp2, sp3
- c)sp3,sp2,sp3,sp3
- d)sp3,sp2,sp,sp3
Correct answer is option 'A'. Can you explain this answer?
Which type of hybridisation of each carbon is there in the compound?
CH3 - CH = CH - CN
CH3 - CH = CH - CN
a)
sp3, sp2, sp2, sp
b)
sp3, sp2, sp2, sp3
c)
sp3,sp2,sp3,sp3
d)
sp3,sp2,sp,sp3
|
|
Yash Majumdar answered |
Understanding Hybridization in CH3 - CH = CH - CN
To determine the hybridization of each carbon in the compound CH3 - CH = CH - CN, we analyze the bonding and geometry around each carbon atom.
1. Carbon 1 (CH3)
- This carbon is bonded to three hydrogen atoms and one other carbon atom.
- The geometry is tetrahedral.
- Hybridization: sp3
2. Carbon 2 (CH)
- This carbon is bonded to one hydrogen atom, one carbon atom (C1), and one carbon atom (C3) through a double bond.
- The geometry is trigonal planar due to the double bond.
- Hybridization: sp2
3. Carbon 3 (CH)
- This carbon is bonded to one hydrogen atom and one carbon atom (C2) through a double bond, and a cyanide group (CN).
- The geometry is also trigonal planar.
- Hybridization: sp2
4. Carbon 4 (CN)
- The carbon in the cyanide group is triple-bonded to nitrogen (N) and is also connected to carbon (C3).
- The geometry is linear due to the triple bond.
- Hybridization: sp
Summary of Hybridization
- Carbon 1: sp3
- Carbon 2: sp2
- Carbon 3: sp2
- Carbon 4: sp
Conclusion
The correct hybridization scheme for the compound CH3 - CH = CH - CN is sp3, sp2, sp2, sp. Thus, the correct answer is option 'A'.
To determine the hybridization of each carbon in the compound CH3 - CH = CH - CN, we analyze the bonding and geometry around each carbon atom.
1. Carbon 1 (CH3)
- This carbon is bonded to three hydrogen atoms and one other carbon atom.
- The geometry is tetrahedral.
- Hybridization: sp3
2. Carbon 2 (CH)
- This carbon is bonded to one hydrogen atom, one carbon atom (C1), and one carbon atom (C3) through a double bond.
- The geometry is trigonal planar due to the double bond.
- Hybridization: sp2
3. Carbon 3 (CH)
- This carbon is bonded to one hydrogen atom and one carbon atom (C2) through a double bond, and a cyanide group (CN).
- The geometry is also trigonal planar.
- Hybridization: sp2
4. Carbon 4 (CN)
- The carbon in the cyanide group is triple-bonded to nitrogen (N) and is also connected to carbon (C3).
- The geometry is linear due to the triple bond.
- Hybridization: sp
Summary of Hybridization
- Carbon 1: sp3
- Carbon 2: sp2
- Carbon 3: sp2
- Carbon 4: sp
Conclusion
The correct hybridization scheme for the compound CH3 - CH = CH - CN is sp3, sp2, sp2, sp. Thus, the correct answer is option 'A'.
Match the following:
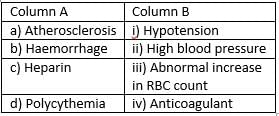
- a)a)-i, b)-iii, c)-iv, d)-ii
- b)a)-iv, b)-i, c)-ii, d)-iii
- c)a)-iii, b)-iv, c)-ii, d)-i
- d)a)-ii, b)-i, c)-iv, d)-iii
Correct answer is option 'D'. Can you explain this answer?
Match the following:


a)
a)-i, b)-iii, c)-iv, d)-ii
b)
a)-iv, b)-i, c)-ii, d)-iii
c)
a)-iii, b)-iv, c)-ii, d)-i
d)
a)-ii, b)-i, c)-iv, d)-iii

|
Rajeev Sharma answered |
Antherosclerosis leads to high blood pressure, haemorrhage causes hypotension, heparin is used as anticoagulant and polycythemia causes abnormal increase in RBC count.
Which of these organs are situated in the thoracic cavity?- a)Heart
- b)Kidney
- c)Stomach
- d)Ovaries
Correct answer is option 'A'. Can you explain this answer?
Which of these organs are situated in the thoracic cavity?
a)
Heart
b)
Kidney
c)
Stomach
d)
Ovaries

|
EduRev NEET answered |
- The muscular heart is located in the thoracic cavity of the body.
- It is located on the ventral side of the body.
- The stomach, the kidneys and the ovaries are located below the heart and the lungs.
When a person suffers from chest pain :
i. the reason for this suffering is decreased blood flow and simultaneously lowered oxygen supply to an area of the heart.
ii. deprived of oxygen, myocardium opts for alternative pathways i.e. anaerobic respiration and its byproduct ethanol causes the pain.- a)both are correct
- b)Statement i) is wrong and ii) is correct.
- c)both are wrong
- d)Statement i) is correct and ii) is wrong.
Correct answer is option 'D'. Can you explain this answer?
When a person suffers from chest pain :
i. the reason for this suffering is decreased blood flow and simultaneously lowered oxygen supply to an area of the heart.
ii. deprived of oxygen, myocardium opts for alternative pathways i.e. anaerobic respiration and its byproduct ethanol causes the pain.
i. the reason for this suffering is decreased blood flow and simultaneously lowered oxygen supply to an area of the heart.
ii. deprived of oxygen, myocardium opts for alternative pathways i.e. anaerobic respiration and its byproduct ethanol causes the pain.
a)
both are correct
b)
Statement i) is wrong and ii) is correct.
c)
both are wrong
d)
Statement i) is correct and ii) is wrong.

|
Nilotpal Gupta answered |
A symptom of acute chest pain appears when no enough oxygen is reaching the heart muscle.
In our blood :
i. Granulocytes which constitutes maximum percentage of total leucocytes are neutrophils.
ii. Elevated number of neutrophils in blood indicates an acute infection such as appendicitis.- a)both are wrong
- b)Statement i) is correct and ii) is wrong
- c)both are correct
- d)Statement i) is wrong and ii) is correct
Correct answer is option 'C'. Can you explain this answer?
In our blood :
i. Granulocytes which constitutes maximum percentage of total leucocytes are neutrophils.
ii. Elevated number of neutrophils in blood indicates an acute infection such as appendicitis.
i. Granulocytes which constitutes maximum percentage of total leucocytes are neutrophils.
ii. Elevated number of neutrophils in blood indicates an acute infection such as appendicitis.
a)
both are wrong
b)
Statement i) is correct and ii) is wrong
c)
both are correct
d)
Statement i) is wrong and ii) is correct

|
Krish Chakraborty answered |
In human blood, granulocytes and agranulocytes types of leucocytes are present. Neutrophils constitute the maximum percentage of total WBCs. Sharp increase in number of neutrophils in blood indicates an acute infection such as appendicitis.
As Soham, ages with chronic CAD the lumen of his coronary artery may get nearly completely obstructed Result is flow of blood to heart tissue is restricted such that the tissue receives inadequate supply of oxygen rich blood and this condition is diagnosed as :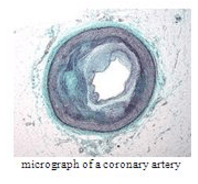
- a)Heart failure
- b)Hypertension
- c)Myocardial ischemia
- d)Myocardial infarction
Correct answer is option 'C'. Can you explain this answer?
As Soham, ages with chronic CAD the lumen of his coronary artery may get nearly completely obstructed Result is flow of blood to heart tissue is restricted such that the tissue receives inadequate supply of oxygen rich blood and this condition is diagnosed as :
a)
Heart failure
b)
Hypertension
c)
Myocardial ischemia
d)
Myocardial infarction

|
Kunal Rane answered |
In case of chronic CAD, the lumen of coronary artery may get nearly completely obstructed that results into restricted flow of blood to heart. The tissue receives inadequate bloods called myocardial ischemia.
Chapter doubts & questions for December Week 3 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of December Week 3 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily