All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of April Week 4 for NEET Exam
The cells of endosperm have 24 chromosomes. What will be the number of chromosomes in the gametes? - a)8
- b)16
- c)23
- d)32
Correct answer is option 'A'. Can you explain this answer?
The cells of endosperm have 24 chromosomes. What will be the number of chromosomes in the gametes?
a)
8
b)
16
c)
23
d)
32
|
|
Mira Joshi answered |
In angiosperms, endosperm is a triploid structure.
∴ 3n = 24 (given), then n = 8
As gametes are haploid structures, therefore number of chromosomes in gametes is 8
∴ 3n = 24 (given), then n = 8
As gametes are haploid structures, therefore number of chromosomes in gametes is 8
Which among the following molecule is not a dipole?- a)NH3
- b)H2O
- c)HCl
- d)CH4
Correct answer is option 'D'. Can you explain this answer?
Which among the following molecule is not a dipole?
a)
NH3
b)
H2O
c)
HCl
d)
CH4
|
|
Krish Kapoor answered |
Explanation:
Dipole moment is a measure of the polarity of a molecule. A molecule is considered polar if it has a dipole moment, and nonpolar if it does not have a dipole moment. The dipole moment of a molecule depends on the electronegativity difference between the atoms in the molecule and the geometry of the molecule.
CH4 (Methane) molecule is not a dipole because of the following reasons:
1. Symmetry: The CH4 molecule has a tetrahedral geometry, with the carbon atom at the center and the four hydrogen atoms at the corners of the tetrahedron. The molecule has a perfect tetrahedral symmetry, with the four C-H bonds pointing towards the corners of the tetrahedron. The symmetry of the molecule cancels out the dipole moments of the individual C-H bonds, resulting in a net dipole moment of zero.
2. Electronegativity: The electronegativity difference between carbon and hydrogen is small, and hence, the C-H bonds are nonpolar. The nonpolar nature of the bonds further contributes to the absence of the dipole moment in the molecule.
Therefore, CH4 molecule is not a dipole.
Dipole moment is a measure of the polarity of a molecule. A molecule is considered polar if it has a dipole moment, and nonpolar if it does not have a dipole moment. The dipole moment of a molecule depends on the electronegativity difference between the atoms in the molecule and the geometry of the molecule.
CH4 (Methane) molecule is not a dipole because of the following reasons:
1. Symmetry: The CH4 molecule has a tetrahedral geometry, with the carbon atom at the center and the four hydrogen atoms at the corners of the tetrahedron. The molecule has a perfect tetrahedral symmetry, with the four C-H bonds pointing towards the corners of the tetrahedron. The symmetry of the molecule cancels out the dipole moments of the individual C-H bonds, resulting in a net dipole moment of zero.
2. Electronegativity: The electronegativity difference between carbon and hydrogen is small, and hence, the C-H bonds are nonpolar. The nonpolar nature of the bonds further contributes to the absence of the dipole moment in the molecule.
Therefore, CH4 molecule is not a dipole.
For an ideal solution with pA > pB, which of the following is true?
- a)(xA)liquid = (xB)vapour
- b)(xA)liquid > (xB)vapour
- c)(xA)liquid < (xB)vapour
- d)(xA)liquid and (xB)vapour do not bear any relationship with each other
Correct answer is option 'C'. Can you explain this answer?
For an ideal solution with pA > pB, which of the following is true?
a)
(xA)liquid = (xB)vapour
b)
(xA)liquid > (xB)vapour
c)
(xA)liquid < (xB)vapour
d)
(xA)liquid and (xB)vapour do not bear any relationship with each other
|
|
Dev Patel answered |
Since the vapor pressure of A is more than B so the mole fraction of A is more in vapor phase than liquid phase as A is more volatile hence vapor phase would be richer in A. Thus the correct option is (xA)liquid < (xB)Vapor
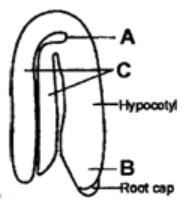
Coleorhiza and coleoptile are the protective sheaths coverging_______and__________respectively.
- a)plumule, epicotyl
- b)plumule, radicle
- c)radicle, plumule
- d)radicle, hypocotyl
Correct answer is option 'C'. Can you explain this answer?
Coleorhiza and coleoptile are the protective sheaths coverging_______and__________respectively.
a)
plumule, epicotyl
b)
plumule, radicle
c)
radicle, plumule
d)
radicle, hypocotyl
|
|
Vijay Bansal answered |
- The epicotyl bearing shoot apex and leaf primordia are enclosed in a foliar structure called coleoptile. It protects the plumule during emergence from the soil.
- The radicle is protected in a sheath called coleorhiza. It protects the radicle during its passage into the soil.
- So, the correct answer is " radicle, plumule ".
If the force acting on a point charge kept on the axis of an electric dipole is F, what will be the amount of force if the distance of the point charge is doubled from the dipole?- a)2F
- b)F/8
- c)F/2
- d)F/4
Correct answer is option 'B'. Can you explain this answer?
If the force acting on a point charge kept on the axis of an electric dipole is F, what will be the amount of force if the distance of the point charge is doubled from the dipole?
a)
2F
b)
F/8
c)
F/2
d)
F/4
|
|
Sanaya Kumar answered |
Given:
Force acting on a point charge kept on the axis of an electric dipole = F
Distance of the point charge from the dipole = d
If the distance of the point charge is doubled from the dipole, then the new distance = 2d
To find:
The amount of force when the distance of the point charge is doubled from the dipole.
Solution:
Electric dipole is a pair of equal and opposite charges separated by a distance 'd'. The direction of the dipole moment is from negative to positive charge.
The electric field due to an electric dipole at a point on its axial line at a distance 'r' from the center of the dipole is given by:
E = 2kp/(r^3)
where k is the Coulomb constant, p is the magnitude of the electric dipole moment and r is the distance from the center of the dipole.
The force experienced by a point charge 'q' placed in an electric field 'E' is given by:
F = qE
In this case, the force acting on the point charge due to the electric field of the dipole is given by:
F = q * 2kp/(d^3)
When the distance of the point charge is doubled from the dipole, the new distance becomes 2d. The electric field due to the dipole at this new distance is given by:
E' = 2kp/(8d^3) = kp/(4d^3)
The force experienced by the point charge at this new distance is given by:
F' = q * kp/(4d^3)
Therefore, the ratio of the new force to the original force is:
F'/F = (q * kp/(4d^3)) / (q * 2kp/(d^3)) = 1/8
Hence, the amount of force when the distance of the point charge is doubled from the dipole is F/8. Therefore, the correct option is B.
Force acting on a point charge kept on the axis of an electric dipole = F
Distance of the point charge from the dipole = d
If the distance of the point charge is doubled from the dipole, then the new distance = 2d
To find:
The amount of force when the distance of the point charge is doubled from the dipole.
Solution:
Electric dipole is a pair of equal and opposite charges separated by a distance 'd'. The direction of the dipole moment is from negative to positive charge.
The electric field due to an electric dipole at a point on its axial line at a distance 'r' from the center of the dipole is given by:
E = 2kp/(r^3)
where k is the Coulomb constant, p is the magnitude of the electric dipole moment and r is the distance from the center of the dipole.
The force experienced by a point charge 'q' placed in an electric field 'E' is given by:
F = qE
In this case, the force acting on the point charge due to the electric field of the dipole is given by:
F = q * 2kp/(d^3)
When the distance of the point charge is doubled from the dipole, the new distance becomes 2d. The electric field due to the dipole at this new distance is given by:
E' = 2kp/(8d^3) = kp/(4d^3)
The force experienced by the point charge at this new distance is given by:
F' = q * kp/(4d^3)
Therefore, the ratio of the new force to the original force is:
F'/F = (q * kp/(4d^3)) / (q * 2kp/(d^3)) = 1/8
Hence, the amount of force when the distance of the point charge is doubled from the dipole is F/8. Therefore, the correct option is B.
In the most common type of endosperm development:- a)cell wall formation occurs simultaneously with each nuclear division of PEN from the beginning.
- b) the PEN undergoes successive nuclear divisions to give rise to free nuclei and subsequently cell wall formation occurs.
- c)the PEN undergoes one division without cell wall formation and then all subsequent divisions are associated with cell wall formation.
- d)cell wall formation does not take place at all.
Correct answer is option 'B'. Can you explain this answer?
In the most common type of endosperm development:
a)
cell wall formation occurs simultaneously with each nuclear division of PEN from the beginning.
b)
the PEN undergoes successive nuclear divisions to give rise to free nuclei and subsequently cell wall formation occurs.
c)
the PEN undergoes one division without cell wall formation and then all subsequent divisions are associated with cell wall formation.
d)
cell wall formation does not take place at all.

|
Ciel Knowledge answered |
In the most common type of endosperm development:
- The PEN undergoes successive nuclear divisions to give rise to free nuclei.
- Subsequently, cell wall formation occurs.
This process implies that the initial divisions of the PEN do not involve cell wall formation; instead, the formation of free nuclei precedes cell wall development. This sequence is a fundamental aspect of endosperm development, ensuring the creation of a structured cellular framework during this critical stage of plant growth.
- The PEN undergoes successive nuclear divisions to give rise to free nuclei.
- Subsequently, cell wall formation occurs.
This process implies that the initial divisions of the PEN do not involve cell wall formation; instead, the formation of free nuclei precedes cell wall development. This sequence is a fundamental aspect of endosperm development, ensuring the creation of a structured cellular framework during this critical stage of plant growth.
Persistent nucellus is called as______________and is found in __________.- a)perisperm, black pepper
- b)perisperm, groundnut
- c)endosperm, black pepper
- d)endosperm,groundnut
Correct answer is option 'A'. Can you explain this answer?
Persistent nucellus is called as______________and is found in __________.
a)
perisperm, black pepper
b)
perisperm, groundnut
c)
endosperm, black pepper
d)
endosperm,groundnut
|
|
Mira Joshi answered |
In some seeds, remains of nucellus persist. This residual nucellus which persists in the seed is called perisperm, e.g., black pepper, coffee, castor, cardamum, Nymphaea.
If an electric dipole is placed in a non-uniform electric field ______ will act on the dipole.- a)A force but no torque
- b)Both force and torque
- c)Torque but no force
- d)No torque or force
Correct answer is option 'B'. Can you explain this answer?
If an electric dipole is placed in a non-uniform electric field ______ will act on the dipole.
a)
A force but no torque
b)
Both force and torque
c)
Torque but no force
d)
No torque or force
|
|
Neha Sharma answered |
In the case of a non-uniform electric field, the force acting on both the charges of the dipole will be unequal. So, there will be a net force acting on the dipole in a certain direction. Also, there will be a torque due to two forces acting at two different points. But in case of a uniform electric field, the net force on the dipole will be zero but net torque will be non-zero.
Given below are few mixtures formed by mixing two components. Which of the following binary mixtures will have same composition in liquid and vapour phase?
(i) Ethanol + Chloroform
(ii) Nitric acid + Water
(iii) Benzene + Toluene
(iv) Ethyl chloride + Ethyl bromide
- a)(i) and (iii)
- b)(iii) and (iv)
- c)(i), (ii) and (iii)
- d)(i) and (ii)
Correct answer is option 'B'. Can you explain this answer?
Given below are few mixtures formed by mixing two components. Which of the following binary mixtures will have same composition in liquid and vapour phase?
(i) Ethanol + Chloroform
(ii) Nitric acid + Water
(iii) Benzene + Toluene
(iv) Ethyl chloride + Ethyl bromide
(i) Ethanol + Chloroform
(ii) Nitric acid + Water
(iii) Benzene + Toluene
(iv) Ethyl chloride + Ethyl bromide
a)
(i) and (iii)
b)
(iii) and (iv)
c)
(i), (ii) and (iii)
d)
(i) and (ii)
|
|
Rohit Jain answered |
(iii) and (iv) will form ideal solutions hence do not form azeotropes. Azeotropes have same composition in liquid and vapour form when distilled.
To determine which binary mixtures will have the same composition in the liquid and vapor phases, we need to identify mixtures that form ideal solutions. In an ideal solution, the composition of the liquid phase and the vapor phase is the same at equilibrium.
Here’s a brief analysis of each mixture:
-
Ethanol + Chloroform:
- This mixture does not behave ideally due to strong hydrogen bonding interactions between ethanol and chloroform, which can cause deviations from Raoult's Law.
-
Nitric Acid + Water:
- Nitric acid and water form a non-ideal solution. Nitric acid forms strong hydrogen bonds with water, resulting in significant deviations from ideal behavior. Thus, the composition in the liquid and vapor phases will not be the same.
-
Benzene + Toluene:
- Benzene and toluene form an ideal solution. The interactions between benzene and toluene are similar, and thus the composition of the liquid and vapor phases will be the same.
-
Ethyl Chloride + Ethyl Bromide:
- Ethyl chloride and ethyl bromide also form an ideal solution. The interactions between these two similar substances lead to minimal deviations from ideal behavior.
Based on the above analyses, the mixtures that will have the same composition in the liquid and vapor phases are:
2. (iii) and (iv)
So the correct answer is:
2. (iii) and (iv)
Dipole moment depends on _______- a)Charge only
- b)Charge and length of a dipole
- c)Charge, length of a dipole and dielectric constant of the medium
- d)Charge and dielectric constant of the medium
Correct answer is option 'B'. Can you explain this answer?
Dipole moment depends on _______
a)
Charge only
b)
Charge and length of a dipole
c)
Charge, length of a dipole and dielectric constant of the medium
d)
Charge and dielectric constant of the medium
|
|
Amar Das answered |
Dipole moment is a measure of the polarity of a molecule. It is defined as the product of the magnitude of the charge on either end of a polar molecule and the distance between them. The dipole moment depends on the following factors:
Charge: The magnitude of the charge on either end of a polar molecule determines the strength of the dipole moment. A larger charge will result in a larger dipole moment.
Length of a dipole: The distance between the charges on either end of a polar molecule determines the length of the dipole. A longer distance will result in a larger dipole moment.
Dielectric constant of the medium: The dielectric constant of the medium in which the dipole is placed will affect the strength of the dipole moment. A higher dielectric constant will result in a larger dipole moment.
Therefore, the correct answer is option B, which states that the dipole moment depends on the charge and length of a dipole. The dielectric constant of the medium can affect the strength of the dipole moment, but it is not a primary factor.
Charge: The magnitude of the charge on either end of a polar molecule determines the strength of the dipole moment. A larger charge will result in a larger dipole moment.
Length of a dipole: The distance between the charges on either end of a polar molecule determines the length of the dipole. A longer distance will result in a larger dipole moment.
Dielectric constant of the medium: The dielectric constant of the medium in which the dipole is placed will affect the strength of the dipole moment. A higher dielectric constant will result in a larger dipole moment.
Therefore, the correct answer is option B, which states that the dipole moment depends on the charge and length of a dipole. The dielectric constant of the medium can affect the strength of the dipole moment, but it is not a primary factor.
Which of the following solutions shows positive deviation from Raoult's law?- a)Acetone + Aniline
- b)Acetone + Ethanol
- c)Water + Nitric acid
- d)Chloroform + Benzene
Correct answer is option 'B'. Can you explain this answer?
Which of the following solutions shows positive deviation from Raoult's law?
a)
Acetone + Aniline
b)
Acetone + Ethanol
c)
Water + Nitric acid
d)
Chloroform + Benzene
|
|
Gaurav Kumar answered |
Acetone + ethanol is an example of solutions showing positive deviation from Raoult's law. Since acetone-ethanol attractions are weaker than acetone - acetone and ethanol-ethanol attractions.
If a non-polar substance is placed in an electric field, what will happen?- a)A net dipole moment will be observed
- b)The substance will oscillate
- c)The substance will orient itself perpendicular to the electric field
- d)It will conduct electricity
Correct answer is option 'A'. Can you explain this answer?
If a non-polar substance is placed in an electric field, what will happen?
a)
A net dipole moment will be observed
b)
The substance will oscillate
c)
The substance will orient itself perpendicular to the electric field
d)
It will conduct electricity
|
|
Priyanka Sharma answered |
A non-polar substance consists of a huge number of dipoles in it, but they are oriented randomly and hence the net dipole moment of the substance becomes 0 and it acts as a non-polar substance. But if it is placed in an electric field, the dipoles present in it will orient themselves in the direction of the field and hence a net dipole moment will be observed, known as induced dipole moment. No current flow or oscillation of the substance will be observed.
When acetone and chloroform are mixed together, hydrogen bonds are formed between them. Which of the following statements is correct about the solution made by mixing acetone and chloroform?- a)On mixing acetone and chloroform will form an ideal solution
- b)On mixing acetone and chloroform positive deviation is shown since the vapour pressure increases
- c)On mixing acetone and chloroform negative deviation is shown since there is decreae in vapour pressure
- d)At a specific composition acetone and chloroform will form minimum boiling azeotrope
Correct answer is option 'C'. Can you explain this answer?
When acetone and chloroform are mixed together, hydrogen bonds are formed between them. Which of the following statements is correct about the solution made by mixing acetone and chloroform?
a)
On mixing acetone and chloroform will form an ideal solution
b)
On mixing acetone and chloroform positive deviation is shown since the vapour pressure increases
c)
On mixing acetone and chloroform negative deviation is shown since there is decreae in vapour pressure
d)
At a specific composition acetone and chloroform will form minimum boiling azeotrope
|
|
Jhanvi Bajaj answered |
Explanation:
When acetone and chloroform are mixed together, hydrogen bonds are formed between them. This interaction between the molecules affects the overall behavior of the solution.
Positive and Negative Deviation:
The behavior of a solution can be classified as either an ideal solution, positive deviation, or negative deviation based on the change in vapor pressure compared to the vapor pressure of the pure components.
- Ideal Solution: An ideal solution is formed when the vapor pressure of the solution is equal to the vapor pressure of the pure components. In an ideal solution, there are no significant interactions between the molecules, and the vapor pressure follows Raoult's law. This means that the partial vapor pressure of each component is directly proportional to its mole fraction in the solution.
- Positive Deviation: A positive deviation occurs when the vapor pressure of the solution is higher than the vapor pressure of the pure components. This indicates that there are attractive interactions between the molecules in the solution that are stronger than the attractive interactions in the pure components. As a result, the vapor pressure of each component is higher than expected based on Raoult's law.
- Negative Deviation: A negative deviation occurs when the vapor pressure of the solution is lower than the vapor pressure of the pure components. This indicates that there are attractive interactions between the molecules in the solution that are weaker than the attractive interactions in the pure components. As a result, the vapor pressure of each component is lower than expected based on Raoult's law.
Explanation of the Correct Answer:
In the case of acetone and chloroform, when they are mixed together, hydrogen bonds are formed between them. The presence of hydrogen bonds results in stronger attractive interactions between the molecules in the solution.
Since hydrogen bonding is a stronger interaction compared to the van der Waals forces present in the pure components, the attractive interactions between acetone and chloroform molecules in the solution are stronger than the attractive interactions in the pure components. This leads to a decrease in the vapor pressure of both acetone and chloroform in the solution compared to their vapor pressures in the pure state.
Therefore, when acetone and chloroform are mixed together, negative deviation is shown since there is a decrease in vapor pressure. The correct answer is option 'C'.
When acetone and chloroform are mixed together, hydrogen bonds are formed between them. This interaction between the molecules affects the overall behavior of the solution.
Positive and Negative Deviation:
The behavior of a solution can be classified as either an ideal solution, positive deviation, or negative deviation based on the change in vapor pressure compared to the vapor pressure of the pure components.
- Ideal Solution: An ideal solution is formed when the vapor pressure of the solution is equal to the vapor pressure of the pure components. In an ideal solution, there are no significant interactions between the molecules, and the vapor pressure follows Raoult's law. This means that the partial vapor pressure of each component is directly proportional to its mole fraction in the solution.
- Positive Deviation: A positive deviation occurs when the vapor pressure of the solution is higher than the vapor pressure of the pure components. This indicates that there are attractive interactions between the molecules in the solution that are stronger than the attractive interactions in the pure components. As a result, the vapor pressure of each component is higher than expected based on Raoult's law.
- Negative Deviation: A negative deviation occurs when the vapor pressure of the solution is lower than the vapor pressure of the pure components. This indicates that there are attractive interactions between the molecules in the solution that are weaker than the attractive interactions in the pure components. As a result, the vapor pressure of each component is lower than expected based on Raoult's law.
Explanation of the Correct Answer:
In the case of acetone and chloroform, when they are mixed together, hydrogen bonds are formed between them. The presence of hydrogen bonds results in stronger attractive interactions between the molecules in the solution.
Since hydrogen bonding is a stronger interaction compared to the van der Waals forces present in the pure components, the attractive interactions between acetone and chloroform molecules in the solution are stronger than the attractive interactions in the pure components. This leads to a decrease in the vapor pressure of both acetone and chloroform in the solution compared to their vapor pressures in the pure state.
Therefore, when acetone and chloroform are mixed together, negative deviation is shown since there is a decrease in vapor pressure. The correct answer is option 'C'.
What are the conditions for an ideal solution which obeys Raoult's law over the entire range of concentration?- a)ΔmixH = 0, ΔmixV = 0, PTotal = pAoxA + pBoxB
- b)ΔmixH = +ve, ΔmixV = 0, PTotal = pAoxA + pBoxB
- c)ΔmixH = 0, ΔmixV = +ve, PTotal = pAoxA + pBoxB
- d)ΔmixH = 0, ΔmixV = 0, PTotal = pBoxB
Correct answer is option 'A'. Can you explain this answer?
What are the conditions for an ideal solution which obeys Raoult's law over the entire range of concentration?
a)
ΔmixH = 0, ΔmixV = 0, PTotal = pAoxA + pBoxB
b)
ΔmixH = +ve, ΔmixV = 0, PTotal = pAoxA + pBoxB
c)
ΔmixH = 0, ΔmixV = +ve, PTotal = pAoxA + pBoxB
d)
ΔmixH = 0, ΔmixV = 0, PTotal = pBoxB
|
|
Jyoti Sengupta answered |
For an ideal solution, ΔH and Delta ΔV for mixing should be zero. PTotal = pA + pB and A − A, B − B and A − B interactions are nearly same.
The system that forms maximum boiling azeotrope is- a)Acetone - chloroform
- b)Ethanol - acetone
- c)n-hexane - n- heptane
- d)Carbon disulphide - acetone
Correct answer is option 'A'. Can you explain this answer?
The system that forms maximum boiling azeotrope is
a)
Acetone - chloroform
b)
Ethanol - acetone
c)
n-hexane - n- heptane
d)
Carbon disulphide - acetone
|
|
Muskaan Iyer answered |
Understanding Maximum Boiling Azeotropes
Azeotropes are mixtures of two or more liquids that boil at a constant temperature and maintain the same composition in both liquid and vapor phases. They can be classified into maximum boiling and minimum boiling azeotropes.
Maximum Boiling Azeotropes
- A maximum boiling azeotrope is formed when the boiling point of the mixture is higher than that of any of its components.
- This phenomenon occurs due to strong intermolecular forces between the components, leading to a higher boiling point.
Acetone - Chloroform Azeotrope
- The acetone-chloroform mixture exhibits a maximum boiling azeotropic behavior.
- In this system, strong hydrogen bonding and dipole-dipole interactions create a stable structure that elevates the boiling point.
- The azeotropic mixture has a boiling point higher than either pure acetone or chloroform, making it a classic example of a maximum boiling azeotrope.
Comparison with Other Options
- Ethanol - Acetone: This combination forms a minimum boiling azeotrope due to weaker interactions.
- n-Hexane - n-Heptane: These are non-polar hydrocarbons that do not exhibit azeotropic behavior.
- Carbon Disulphide - Acetone: This mixture also does not form a maximum boiling azeotrope.
Conclusion
The correct answer is option 'A' (Acetone - Chloroform) as it exemplifies the characteristics of a maximum boiling azeotrope with a significantly elevated boiling point compared to its components. Understanding such systems is crucial in distillation and separation processes in chemistry.
Azeotropes are mixtures of two or more liquids that boil at a constant temperature and maintain the same composition in both liquid and vapor phases. They can be classified into maximum boiling and minimum boiling azeotropes.
Maximum Boiling Azeotropes
- A maximum boiling azeotrope is formed when the boiling point of the mixture is higher than that of any of its components.
- This phenomenon occurs due to strong intermolecular forces between the components, leading to a higher boiling point.
Acetone - Chloroform Azeotrope
- The acetone-chloroform mixture exhibits a maximum boiling azeotropic behavior.
- In this system, strong hydrogen bonding and dipole-dipole interactions create a stable structure that elevates the boiling point.
- The azeotropic mixture has a boiling point higher than either pure acetone or chloroform, making it a classic example of a maximum boiling azeotrope.
Comparison with Other Options
- Ethanol - Acetone: This combination forms a minimum boiling azeotrope due to weaker interactions.
- n-Hexane - n-Heptane: These are non-polar hydrocarbons that do not exhibit azeotropic behavior.
- Carbon Disulphide - Acetone: This mixture also does not form a maximum boiling azeotrope.
Conclusion
The correct answer is option 'A' (Acetone - Chloroform) as it exemplifies the characteristics of a maximum boiling azeotrope with a significantly elevated boiling point compared to its components. Understanding such systems is crucial in distillation and separation processes in chemistry.
What is the dimension of the dipole moment?- a)[L T I]
- b)[L T I2]
- c)[M L T I]
- d)[L T I-1]
Correct answer is option 'A'. Can you explain this answer?
What is the dimension of the dipole moment?
a)
[L T I]
b)
[L T I2]
c)
[M L T I]
d)
[L T I-1]
|
|
Rajesh Gupta answered |
The dipole moment is defined as the product of a charge and distance. The dimension of charge (current*time) is [I T] and the dimension of distance is [L]. Therefore the dimension of dipole moment is [L T I]. Its unit in the CGS and the SI system are esu*cm and C*m respectively.
An electric dipole is placed inside a cube. What will be the nature of electric flux from the cube surface?- a)Coming out of the surface
- b)Coming in towards the surface
- c)No flux at all
- d)Coming out from one half and coming inwards in another half
Correct answer is option 'C'. Can you explain this answer?
An electric dipole is placed inside a cube. What will be the nature of electric flux from the cube surface?
a)
Coming out of the surface
b)
Coming in towards the surface
c)
No flux at all
d)
Coming out from one half and coming inwards in another half
|
|
Shalini Patel answered |
An electric dipole consists of two point charges. The amount of charges is the same but their polarities are different. Therefore the sum of total charges in a dipole is always 0. But flux from a closed surface is related to the total charge inside a surface. As the total charge inside the cube is zero, so there will be no flux coming out or going in towards the surface.
Which of the following solutions is an example of negative deviation from Raoult's law?- a)Acetone + Ethanol
- b)Carbon tetrachloride + Chloroform
- c)Acetone + Chloroform
- d)Water + Ethanol
Correct answer is option 'C'. Can you explain this answer?
Which of the following solutions is an example of negative deviation from Raoult's law?
a)
Acetone + Ethanol
b)
Carbon tetrachloride + Chloroform
c)
Acetone + Chloroform
d)
Water + Ethanol
|
|
Sravya Sarkar answered |
Explanation:
In order to understand this question, we need to first understand what Raoult's law is. Raoult's law states that the partial pressure of a component of an ideal solution is equal to the product of the vapor pressure of that component in its pure state and its mole fraction in the solution. According to Raoult's law, the vapor pressure of a component in a solution should be directly proportional to its mole fraction.
Negative Deviation from Raoult's Law:
When the vapor pressure of a component in a solution is lower than expected based on Raoult's law, it is known as negative deviation. This means that the molecules of the components in the solution tend to attract each other more strongly than in their pure states. Negative deviation occurs when the intermolecular forces of attraction between the molecules in the solution are stronger than the forces between the molecules of the pure components.
Explanation of the Given Options:
a) Acetone and Ethanol: Both acetone and ethanol are polar compounds and they have similar intermolecular forces of attraction. Therefore, they are likely to follow Raoult's law and show positive deviation.
b) Carbon tetrachloride and Chloroform: Carbon tetrachloride and chloroform are nonpolar compounds and they have similar intermolecular forces of attraction. Therefore, they are likely to follow Raoult's law and show positive deviation.
c) Acetone and Chloroform:
Acetone is a polar compound, while chloroform is a nonpolar compound. They have different intermolecular forces of attraction. Acetone has dipole-dipole interactions, while chloroform has London dispersion forces. These different intermolecular forces can lead to negative deviation from Raoult's law.
In the solution of acetone and chloroform, the acetone molecules will have a stronger attraction towards each other due to dipole-dipole interactions. Similarly, the chloroform molecules will have a stronger attraction towards each other due to London dispersion forces. These stronger intermolecular forces will reduce the vapor pressure of both acetone and chloroform in the solution, leading to negative deviation from Raoult's law.
Therefore, option c) Acetone and Chloroform is an example of negative deviation from Raoult's law.
In order to understand this question, we need to first understand what Raoult's law is. Raoult's law states that the partial pressure of a component of an ideal solution is equal to the product of the vapor pressure of that component in its pure state and its mole fraction in the solution. According to Raoult's law, the vapor pressure of a component in a solution should be directly proportional to its mole fraction.
Negative Deviation from Raoult's Law:
When the vapor pressure of a component in a solution is lower than expected based on Raoult's law, it is known as negative deviation. This means that the molecules of the components in the solution tend to attract each other more strongly than in their pure states. Negative deviation occurs when the intermolecular forces of attraction between the molecules in the solution are stronger than the forces between the molecules of the pure components.
Explanation of the Given Options:
a) Acetone and Ethanol: Both acetone and ethanol are polar compounds and they have similar intermolecular forces of attraction. Therefore, they are likely to follow Raoult's law and show positive deviation.
b) Carbon tetrachloride and Chloroform: Carbon tetrachloride and chloroform are nonpolar compounds and they have similar intermolecular forces of attraction. Therefore, they are likely to follow Raoult's law and show positive deviation.
c) Acetone and Chloroform:
Acetone is a polar compound, while chloroform is a nonpolar compound. They have different intermolecular forces of attraction. Acetone has dipole-dipole interactions, while chloroform has London dispersion forces. These different intermolecular forces can lead to negative deviation from Raoult's law.
In the solution of acetone and chloroform, the acetone molecules will have a stronger attraction towards each other due to dipole-dipole interactions. Similarly, the chloroform molecules will have a stronger attraction towards each other due to London dispersion forces. These stronger intermolecular forces will reduce the vapor pressure of both acetone and chloroform in the solution, leading to negative deviation from Raoult's law.
Therefore, option c) Acetone and Chloroform is an example of negative deviation from Raoult's law.
Intermolecular forces between n-hexane and n-heptane are nearly same as between hexane and heptane individually. When these two are mixed, which of the following is not true about the solution formed?- a)It obeys Raoult’s law, i.e. pA = xAp∘A and pB = xBp∘B
- b)ΔHmixing is zero
- c)ΔVmixing is zero
- d)It forms minimum boiling azeotrope
Correct answer is option 'D'. Can you explain this answer?
Intermolecular forces between n-hexane and n-heptane are nearly same as between hexane and heptane individually. When these two are mixed, which of the following is not true about the solution formed?
a)
It obeys Raoult’s law, i.e. pA = xAp∘A and pB = xBp∘B
b)
ΔHmixing is zero
c)
ΔVmixing is zero
d)
It forms minimum boiling azeotrope
|
|
Ananya Das answered |
Azeotropes are formed by the solutions which show deviations from ideal behaviour.
Endosperm development precedes ____ development.- a)pollen tube
- b)embryo
- c)nuclei
- d)micropyle
Correct answer is option 'B'. Can you explain this answer?
Endosperm development precedes ____ development.
a)
pollen tube
b)
embryo
c)
nuclei
d)
micropyle

|
Lead Academy answered |
- Following double fertilization, post fertilization events begin.
- Events like endosperm formation and embryo development, formation of seeds from ovules and ovary into fruit are classified as post fertilization events.
Identify the wrong statement regarding post fertilization development.- a)The ovary wall develops into pericarp
- b)The outer integument of the ovule develops into tegmen
- c)The fusion nucleus (triple nucleus) develops into endosperm
- d)The ovule develops into seed
Correct answer is option 'B'. Can you explain this answer?
Identify the wrong statement regarding post fertilization development.
a)
The ovary wall develops into pericarp
b)
The outer integument of the ovule develops into tegmen
c)
The fusion nucleus (triple nucleus) develops into endosperm
d)
The ovule develops into seed
|
|
Mira Joshi answered |
After fertilisation, the integuments of the ovule form the protective coats (seed coats). Outer integument develops into testa and the inner one develops into tegmen.
1uC and -1uC are placed at a distance of 5 cm forming a dipole. What is the amount of torque required to place the dipole perpendicularly to an electric field of 3*105 N/C?- a)5*10-3 N. m
- b)15*10-3 N. m
- c)1*10-3 N. m
- d)10*10-3 N. m
Correct answer is option 'B'. Can you explain this answer?
1uC and -1uC are placed at a distance of 5 cm forming a dipole. What is the amount of torque required to place the dipole perpendicularly to an electric field of 3*105 N/C?
a)
5*10-3 N. m
b)
15*10-3 N. m
c)
1*10-3 N. m
d)
10*10-3 N. m
|
|
Suresh Iyer answered |
Dipole moment of the dipole = 1*10-6*5*10-2 C. m=5*10-8 C. m. Given the electric field-intensity is E=3*105 N/C. Therefore required torque will be p*E*sinθ where θ is the angle between the electric field and the dipole moment = 90 degrees. Therefore torque required in this case will be 5 *10-8*3*105*sin 90 N. m=15*10-3 N. m.
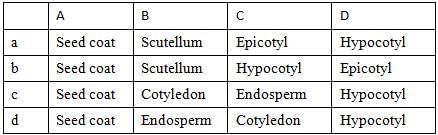
Identify the parts labelled as A, B, C and D in the given figure and select the correct option from the codes given below
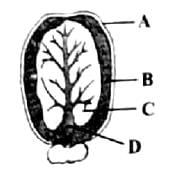
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
Identify the parts labelled as A, B, C and D in the given figure and select the correct option from the codes given below


a)
a
b)
b
c)
c
d)
d
|
|
Mira Joshi answered |
Correct option is D. A-Seed coat, B-Endosperm, C-Cotyledon, D-Hypocotyl
The diagram shows stages in embryo development in a dicot where A, B, and C respectively are: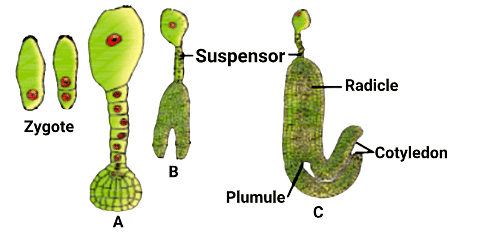
- a)Globular embryo, Heart shaped embryo, Immature Embryo
- b)Globular embryo, Heart shaped embryo, Mature Embryo
- c)Heart-shaped embryo, Globular embryo, Mature Embryo
- d)Heart-shaped embryo, Globular embryo, Immature Embryo
Correct answer is option 'B'. Can you explain this answer?
The diagram shows stages in embryo development in a dicot where A, B, and C respectively are:

a)
Globular embryo, Heart shaped embryo, Immature Embryo
b)
Globular embryo, Heart shaped embryo, Mature Embryo
c)
Heart-shaped embryo, Globular embryo, Mature Embryo
d)
Heart-shaped embryo, Globular embryo, Immature Embryo

|
Ciel Knowledge answered |
(B) The correct answer is B: Globular embryo, Heart-shaped embryo, Mature Embryo.
- Globular embryo: At this stage, the embryo is spherical in shape.
- Heart-shaped embryo: The embryo develops into a heart shape as it matures.
- Mature Embryo: This is the final stage where the embryo is fully developed and ready for seed formation.
Understanding these stages helps track the growth of a dicot embryo from its early spherical form to its mature, seed-forming stage.Globular embryo, Heart shaped embryo, Mature Embryo
- Globular embryo: At this stage, the embryo is spherical in shape.
- Heart-shaped embryo: The embryo develops into a heart shape as it matures.
- Mature Embryo: This is the final stage where the embryo is fully developed and ready for seed formation.
Understanding these stages helps track the growth of a dicot embryo from its early spherical form to its mature, seed-forming stage.Globular embryo, Heart shaped embryo, Mature Embryo
An electric dipole will be in stable equilibrium if the angle between the axis of the dipole and the electric field is ________- a)0 degree
- b)180 degree
- c)90 degree
- d)45 degree
Correct answer is option 'A'. Can you explain this answer?
An electric dipole will be in stable equilibrium if the angle between the axis of the dipole and the electric field is ________
a)
0 degree
b)
180 degree
c)
90 degree
d)
45 degree
|
|
Riya Banerjee answered |
Torque acting on a dipole is p*E*sinθ where E is the electric field. Now θ is 0 degree, so sinθ becomes 0 and hence no torque acts on the dipole. So in this case, no force or torque acts on the dipole. Therefore it will be the condition of stable equilibrium. In the case of θ=180 degree, sinθ is also 0 but the condition is known as unstable equilibrium i.e. if we rotate the dipole a bit, it will not come back to its initial position.
When acetone and chloroform are mixed together, which of the following observations is correct?
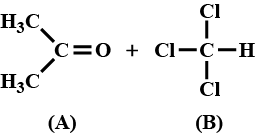
- a)A - A and B - B interactions are stronger than A - B interactions
- b)A - A and B - B interactions are weaker than A - B interactions
- c)A - A and B - B interactions are equal
- d)The liquid form separate layers and are immiscible
Correct answer is option 'B'. Can you explain this answer?
When acetone and chloroform are mixed together, which of the following observations is correct?
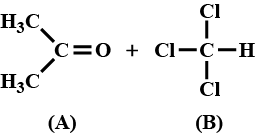
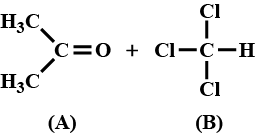
a)
A - A and B - B interactions are stronger than A - B interactions
b)
A - A and B - B interactions are weaker than A - B interactions
c)
A - A and B - B interactions are equal
d)
The liquid form separate layers and are immiscible
|
|
Mira Joshi answered |
When acetone and chloroform are mixed together, a hydrogen bond is formed between them which increases intermolecular interactions. Hence, A − B interactions are stronger than A − A and A − B interactions.
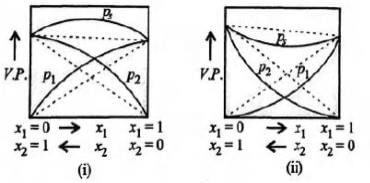
Study the figures given below and mark the correct statement.
- a)(i) Nitric acid + Water,
(ii) Acetone + Ethyl alcohol - b)(i) Water + Ethyl alcohol,
(ii) Acetone + Benzene - c)(i) Acetone + Ethyl alcohol,
(ii) Acetone + Chloroform - d)(i) Benzene + Chloroform,
(ii) Acetone + Chloroform
Correct answer is option 'C'. Can you explain this answer?
Study the figures given below and mark the correct statement.


a)
(i) Nitric acid + Water,
(ii) Acetone + Ethyl alcohol
(ii) Acetone + Ethyl alcohol
b)
(i) Water + Ethyl alcohol,
(ii) Acetone + Benzene
(ii) Acetone + Benzene
c)
(i) Acetone + Ethyl alcohol,
(ii) Acetone + Chloroform
(ii) Acetone + Chloroform
d)
(i) Benzene + Chloroform,
(ii) Acetone + Chloroform
(ii) Acetone + Chloroform
|
|
Lavanya Menon answered |
Acetone + ethyl alcohol solution shows positive deviation while acetone + chloroform shows negative deviation.
Other examples:
Positive deviations - Acetone + ethyl alcohol,
acetone + benzene, water + ethyl alcohol
Negative deviations - Nitric acid + water,
benzene + chloroform
Other examples:
Positive deviations - Acetone + ethyl alcohol,
acetone + benzene, water + ethyl alcohol
Negative deviations - Nitric acid + water,
benzene + chloroform
If an electric dipole is placed in a uniform electric field ______ will act on the dipole.- a)A force but no torque
- b)Both force and torque
- c)Torque but no force
- d)No torque or force
Correct answer is option 'C'. Can you explain this answer?
If an electric dipole is placed in a uniform electric field ______ will act on the dipole.
a)
A force but no torque
b)
Both force and torque
c)
Torque but no force
d)
No torque or force
|
|
Shalini Patel answered |
Dipole is the combination of two equal but opposite charges, kept at a certain distance. If it is placed in a uniform electric field, both the charges will suffer the same but opposite forces on them. As a result, the net force on the dipole becomes zero, but due to equal and opposite forces acting on two different points, there is a net torque acting on the dipole.
In the given diagram, X represents
- a)cellular endosperm
- b)nuclear endosperm
- c)helobial endosperm
- d)ruminate endosperm
Correct answer is option 'B'. Can you explain this answer?
In the given diagram, X represents

a)
cellular endosperm
b)
nuclear endosperm
c)
helobial endosperm
d)
ruminate endosperm
|
|
Mira Joshi answered |
Nuclear endosperm (X) is the most common type of endosperm. It is named so because it contains free nuclei in the beginning. The primary endosperm nucleus divides of free nuclei. Meanwhile central vacuole appears in the central cell and pushes the cytoplasm containing the nuclei to the periphery. The cytoplasm thickens so that the vacuole decreases in size. It ultimately disappears with the exception of a few cases. The multinucleate cytoplasm undergoes cleavage and gives rise to a multicellular tissue, e.g., maize, wheat, rice, sunflower, Capsella bursa-pastoris.
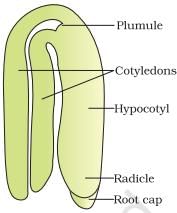
Identify the parts labelled A, B and C in the given figure and select the correct option.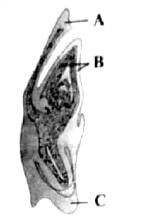
- a)A-Scutellum, B-Coleorhiza, C-Coleoptile
- b)A-Scutellum, B-Coleoptile, C-Coleorhiza
- c)A-Coleoptile, B-Scutellum, C-Coleorhiza
- d)A-Coleorhiza, B-Scutellum, C-Coleoptile
Correct answer is option 'B'. Can you explain this answer?
Identify the parts labelled A, B and C in the given figure and select the correct option.

a)
A-Scutellum, B-Coleorhiza, C-Coleoptile
b)
A-Scutellum, B-Coleoptile, C-Coleorhiza
c)
A-Coleoptile, B-Scutellum, C-Coleorhiza
d)
A-Coleorhiza, B-Scutellum, C-Coleoptile
|
|
Mira Joshi answered |
Given figure represents the monocotyledonous embryo of a grass.
Milk of tender coconut represents (i) and the surrounding white coconut meal represents (ii).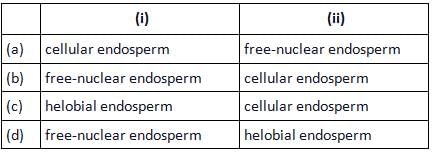
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
Milk of tender coconut represents (i) and the surrounding white coconut meal represents (ii).

a)
a
b)
b
c)
c
d)
d
|
|
Mira Joshi answered |
In coconut (Cocos nucifera), the surrounding white kernel called coconut meal is cellular endosperm and the coconut water (also called coconut milk) in the centre is free nuclear endosperm made up of thousands of nuclei.
Chapter doubts & questions for April Week 4 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of April Week 4 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup