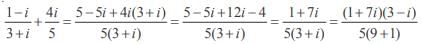All Exams >
JEE >
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 >
All Questions
All questions of Sample Papers for JEE Exam
If the electron in a hydrogen atom jumps from an orbit with level n1 = 2 to an orbit with level n2 = 1 the emitted radiation has a wavelength given by- a)λ = 5/3R
- b)λ = 4/3 R
- c)λ = R/4
- d)λ = 3R/4
Correct answer is option 'B'. Can you explain this answer?
If the electron in a hydrogen atom jumps from an orbit with level n1 = 2 to an orbit with level n2 = 1 the emitted radiation has a wavelength given by
a)
λ = 5/3R
b)
λ = 4/3 R
c)
λ = R/4
d)
λ = 3R/4
|
|
Baishali Pillai answered |
656.3 nm
b) 486.1 nm
c) 434.0 nm
d) 410.2 nm
The correct answer is b) 486.1 nm.
This can be determined using the formula for the wavelength of emitted radiation:
λ = hc/ΔE
where λ is the wavelength, h is Planck's constant, c is the speed of light, and ΔE is the energy difference between the two levels.
The energy difference can be calculated using the formula:
ΔE = Rh(1/n1^2 - 1/n2^2)
where Rh is the Rydberg constant and n1 and n2 are the initial and final energy levels, respectively.
Plugging in the values for n1 and n2, we get:
ΔE = Rh(1/2^2 - 1/1^2) = 3Rh/4
Substituting this into the first formula, we get:
λ = hc/(3Rh/4)
Plugging in the values for h, c, and Rh, we get:
λ = (3 x 10^-7 m)(2.998 x 10^8 m/s)/(3(1.097 x 10^7 m^-1)/4)
λ = 486.1 nm
Therefore, the emitted radiation has a wavelength of 486.1 nm.
b) 486.1 nm
c) 434.0 nm
d) 410.2 nm
The correct answer is b) 486.1 nm.
This can be determined using the formula for the wavelength of emitted radiation:
λ = hc/ΔE
where λ is the wavelength, h is Planck's constant, c is the speed of light, and ΔE is the energy difference between the two levels.
The energy difference can be calculated using the formula:
ΔE = Rh(1/n1^2 - 1/n2^2)
where Rh is the Rydberg constant and n1 and n2 are the initial and final energy levels, respectively.
Plugging in the values for n1 and n2, we get:
ΔE = Rh(1/2^2 - 1/1^2) = 3Rh/4
Substituting this into the first formula, we get:
λ = hc/(3Rh/4)
Plugging in the values for h, c, and Rh, we get:
λ = (3 x 10^-7 m)(2.998 x 10^8 m/s)/(3(1.097 x 10^7 m^-1)/4)
λ = 486.1 nm
Therefore, the emitted radiation has a wavelength of 486.1 nm.
The second law of thermodynamics says that in a cyclic process :- a)work cannot be converted into heat
- b)heat cannot be converted into work
- c)work cannot be completely converted into heat
- d)heat cannot be completely converted into work
Correct answer is option 'D'. Can you explain this answer?
The second law of thermodynamics says that in a cyclic process :
a)
work cannot be converted into heat
b)
heat cannot be converted into work
c)
work cannot be completely converted into heat
d)
heat cannot be completely converted into work
|
|
Sarita Yadav answered |
Because 0 K temperature is unattainable
Using binomial theorem, the value of (0.999)3 correct to 3 decimal places is- a)0.999
- b)0.998
- c)0.997
- d)0.995
Correct answer is option 'C'. Can you explain this answer?
Using binomial theorem, the value of (0.999)3 correct to 3 decimal places is
a)
0.999
b)
0.998
c)
0.997
d)
0.995
|
|
Rohit Jain answered |
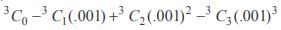
= 1 – .003 + 3 (.000001) – (.000000001) = 0.997
A particle is moving in a straight line. At time t, the distance between the particle from its starting point is given by x = t – 6t2 + t3 . Its acceleration will be zero at- a)t = 1 unit time
- b)t = 2 unit time
- c)t = 3 unit time
- d)t = 4 unit time
Correct answer is option 'B'. Can you explain this answer?
A particle is moving in a straight line. At time t, the distance between the particle from its starting point is given by x = t – 6t2 + t3 . Its acceleration will be zero at
a)
t = 1 unit time
b)
t = 2 unit time
c)
t = 3 unit time
d)
t = 4 unit time
|
|
Varun Kapoor answered |
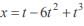
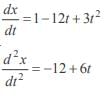
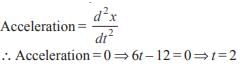
19 g of water at 30° C and 5 g of ice at – 20° C are mixed together in a calorimeter. What is the final temperature of the mixture? Given specific heat of ice = 0.5 cal g–1(°C)–1 and latent heat of fusion of ice = 80 cal g–1- a)0° C
- b)– 5° C
- c)5° C
- d)10° C
Correct answer is option 'C'. Can you explain this answer?
19 g of water at 30° C and 5 g of ice at – 20° C are mixed together in a calorimeter. What is the final temperature of the mixture? Given specific heat of ice = 0.5 cal g–1(°C)–1 and latent heat of fusion of ice = 80 cal g–1
a)
0° C
b)
– 5° C
c)
5° C
d)
10° C
|
|
Kritika Choudhary answered |
°C is mixed with 31 g of water at 50°C. What is the final temperature of the mixture?
To solve this problem, we need to use the principle of conservation of energy:
Q1 + Q2 = Q3
where Q1 is the heat absorbed by the first sample of water, Q2 is the heat absorbed by the second sample of water, and Q3 is the heat released by the final mixture.
We can calculate Q1 and Q2 using the specific heat capacity of water:
Q1 = 19 g x 4.18 J/g·°C x (Tf - 30°C)
Q2 = 31 g x 4.18 J/g·°C x (Tf - 50°C)
where Tf is the final temperature of the mixture.
We can simplify these equations by factorizing out the specific heat capacity and solving for Tf:
Q1 = Q2
19 x (Tf - 30) = 31 x (50 - Tf)
19Tf - 570 = 1550 - 31Tf
50Tf = 2120
Tf = 42.4°C
Therefore, the final temperature of the mixture is 42.4°C.
To solve this problem, we need to use the principle of conservation of energy:
Q1 + Q2 = Q3
where Q1 is the heat absorbed by the first sample of water, Q2 is the heat absorbed by the second sample of water, and Q3 is the heat released by the final mixture.
We can calculate Q1 and Q2 using the specific heat capacity of water:
Q1 = 19 g x 4.18 J/g·°C x (Tf - 30°C)
Q2 = 31 g x 4.18 J/g·°C x (Tf - 50°C)
where Tf is the final temperature of the mixture.
We can simplify these equations by factorizing out the specific heat capacity and solving for Tf:
Q1 = Q2
19 x (Tf - 30) = 31 x (50 - Tf)
19Tf - 570 = 1550 - 31Tf
50Tf = 2120
Tf = 42.4°C
Therefore, the final temperature of the mixture is 42.4°C.
A polygon has 44 diagonals. The number of its sides is- a)10
- b)11
- c)12
- d)13
Correct answer is option 'B'. Can you explain this answer?
A polygon has 44 diagonals. The number of its sides is
a)
10
b)
11
c)
12
d)
13
|
|
Tanuja Kapoor answered |
''C2 – n = 44
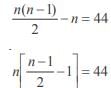
n(n – 3) = 88
n(n – 3) = 11 × 8
n = 11
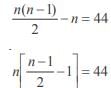
n(n – 3) = 88
n(n – 3) = 11 × 8
n = 11
The equation of the chord of the circle x2 + y2 – 4x = 0 whose mid point is (1, 0) is- a)y = 2
- b)y = 1
- c)x = 2
- d)x = 1
Correct answer is option 'D'. Can you explain this answer?
The equation of the chord of the circle x2 + y2 – 4x = 0 whose mid point is (1, 0) is
a)
y = 2
b)
y = 1
c)
x = 2
d)
x = 1
|
|
Ananya Das answered |

Chord with mid-point (1, 0)
Equation : x = 1
The poisson’s ratio of a material is 0.5. If a force is applied to a wire of this material, there is a decrease in the cross-sectional area by 4%. The percentage increase in the length is :- a)1 %
- b)2%
- c)2.5%
- d)4%
Correct answer is option 'D'. Can you explain this answer?
The poisson’s ratio of a material is 0.5. If a force is applied to a wire of this material, there is a decrease in the cross-sectional area by 4%. The percentage increase in the length is :
a)
1 %
b)
2%
c)
2.5%
d)
4%
|
|
Hansa Sharma answered |
Poisson ratio = 0.5
Therefore density is constant hence change in volume is zero we have
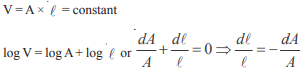
That is 4%
Therefore density is constant hence change in volume is zero we have
That is 4%
. It is difficult to cook rice in an open vessel by boiling it at high altitudes because of- a)low boiling point and high pressure
- b)high boiling point and low pressure
- c)low boiling point and low pressure
- d)high boiling point and high pressure
Correct answer is option 'C'. Can you explain this answer?
. It is difficult to cook rice in an open vessel by boiling it at high altitudes because of
a)
low boiling point and high pressure
b)
high boiling point and low pressure
c)
low boiling point and low pressure
d)
high boiling point and high pressure

|
Devika Banerjee answered |
At high altitude pressure is low and boiling point also low
Which one of the following formulae does not represent an organic compound?- a)C4H10O4
- b)C4H8O4
- c)C4H7 CIO4
- d)C4H9O4
Correct answer is option 'D'. Can you explain this answer?
Which one of the following formulae does not represent an organic compound?
a)
C4H10O4
b)
C4H8O4
c)
C4H7 CIO4
d)
C4H9O4
|
|
Neha Joshi answered |
Unsaturation factor = 0, 1, 1, 0.5 Hence (D)
If α,β be the roots of the quadratic equation x2 + x + 1 = 0 then the equation whose roots are α19 , β7 is- a)x2 – x + 1 = 0
- b)x2 – x – 1 = 0
- c)x2 + x – 1 = 0
- d)x2 + x + 1 = 0
Correct answer is option 'D'. Can you explain this answer?
If α,β be the roots of the quadratic equation x2 + x + 1 = 0 then the equation whose roots are α19 , β7 is
a)
x2 – x + 1 = 0
b)
x2 – x – 1 = 0
c)
x2 + x – 1 = 0
d)
x2 + x + 1 = 0
|
|
Rohit Jain answered |
Roots are ω,ω2
Let α = ω,β = ω2
α 19 = ω,β7 = ω2
∴ Equation remains same i.e. x2 + x+ 1 = 0
Let α = ω,β = ω2
α 19 = ω,β7 = ω2
∴ Equation remains same i.e. x2 + x+ 1 = 0
The general solution of the differential equation 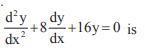
- a)(A + B x)e5x
- b)(A + Bx)e–4x
- c)(A + Bx2 )e4x
- d)(A + Bx4 )e4x
Correct answer is option 'B'. Can you explain this answer?
The general solution of the differential equation 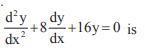
a)
(A + B x)e5x
b)
(A + Bx)e–4x
c)
(A + Bx2 )e4x
d)
(A + Bx4 )e4x
|
|
Anaya Patel answered |
auxilary equation m2 + 8m + 16 = 0 ⇒ m = – 4
Solution
If log3x + log3y = 2 + log32 and log3 (x + y) = 2, then- a)x = 1,y = 8
- b)x = 8, y = 1
- c)x = 3, y = 6
- d)x = 9, y = 3
Correct answer is option 'C'. Can you explain this answer?
If log3x + log3y = 2 + log32 and log3 (x + y) = 2, then
a)
x = 1,y = 8
b)
x = 8, y = 1
c)
x = 3, y = 6
d)
x = 9, y = 3

|
Sahil Singh answered |
We have equation xy = 18 and another equation as x + y = 9
by solving these equation we will get values of x and y
by solving these equation we will get values of x and y
A compound is formed by substitution of two chlorine for two hydrogens in propane. The number of possible isomeric compounds is- a)4
- b)3
- c)5
- d)2
Correct answer is option 'C'. Can you explain this answer?
A compound is formed by substitution of two chlorine for two hydrogens in propane. The number of possible isomeric compounds is
a)
4
b)
3
c)
5
d)
2
|
|
Neha Joshi answered |
Due to presence of chiral carbon compound (IV) is optically active and forms an enantiomer. So total no of isomers =5
If A and B are two matrices such that A+B and AB are both defined, then- a)A and B can be any matrices
- b)A, B are square matrices not necessarily of the same order
- c)A, B are square matrices of the same order
- d)Number of columns of A = number of rows of B
Correct answer is option 'C'. Can you explain this answer?
If A and B are two matrices such that A+B and AB are both defined, then
a)
A and B can be any matrices
b)
A, B are square matrices not necessarily of the same order
c)
A, B are square matrices of the same order
d)
Number of columns of A = number of rows of B
|
|
Lavanya Menon answered |
Addition is defined if order of A is equal to order of B
A B
nxm nxm is defined if m = n
⇒ A, B are square matrices of same order
A B
nxm nxm is defined if m = n
⇒ A, B are square matrices of same order
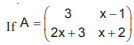 is a symmetric matrix, then the value of x is
is a symmetric matrix, then the value of x is- a)4
- b)3
- c)– 4
- d)– 3
Correct answer is option 'C'. Can you explain this answer?
a)
4
b)
3
c)
– 4
d)
– 3

|
Tulsi Kumari Tulsi Kumar answered |
Since it is a symmetric matrix,
Transpose of A will be equal to A.
This will give eqn 2x+3=x-1.
By solving this eqn , ans will be -4.
Transpose of A will be equal to A.
This will give eqn 2x+3=x-1.
By solving this eqn , ans will be -4.
An organic compound made of C, H and N contains 20% nitrogen. Its molecular weight is :- a)70
- b)140
- c)100
- d)65
Correct answer is option 'A'. Can you explain this answer?
An organic compound made of C, H and N contains 20% nitrogen. Its molecular weight is :
a)
70
b)
140
c)
100
d)
65
|
|
Yash Patel answered |
Nitrogen at. wt. = 14 in a molecule minimum one atom of N is present
i.e., 20% ≡ 14 Molecular weight = 70
100% ≡ 14 × 5 = 70
i.e., 20% ≡ 14 Molecular weight = 70
100% ≡ 14 × 5 = 70
Chapter doubts & questions for Sample Papers - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Sample Papers - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026
3 videos|21 docs|54 tests
|
Related JEE Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily

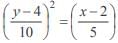
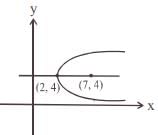


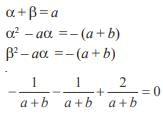
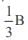
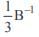
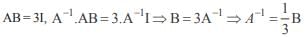

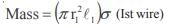
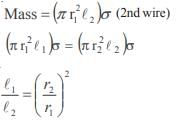
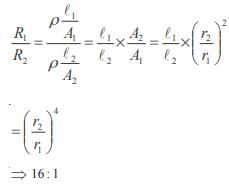
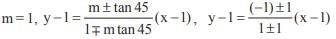
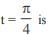
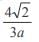
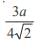
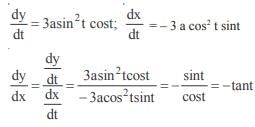
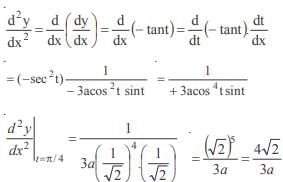
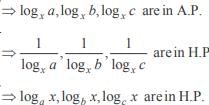


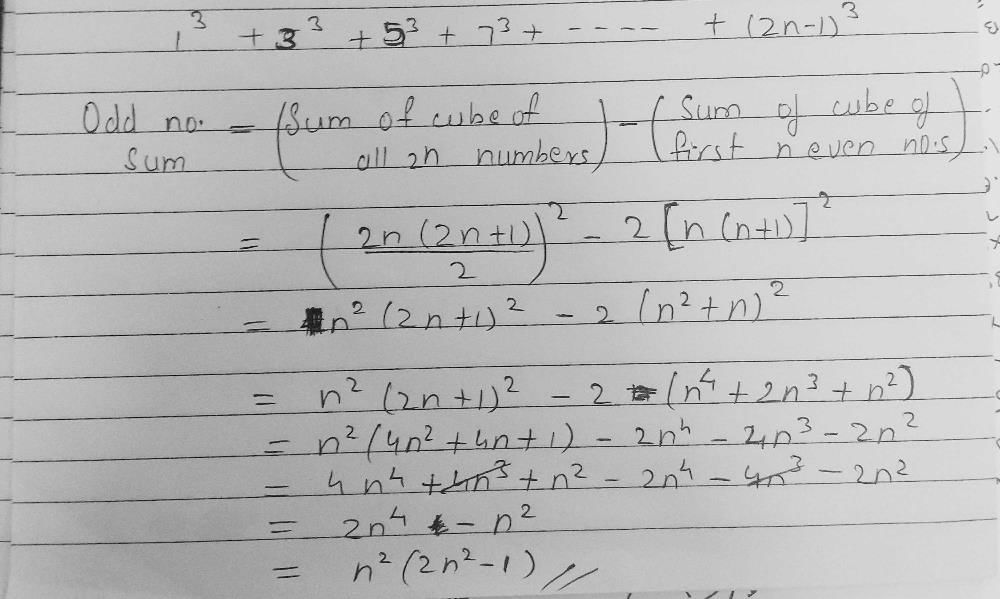
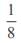
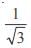
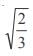
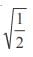
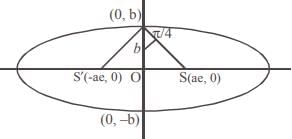
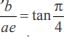
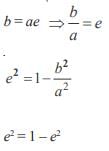
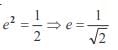
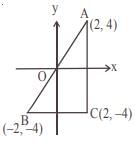
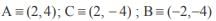
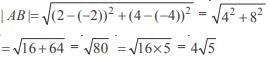
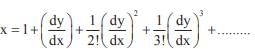
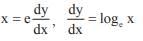

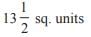
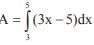
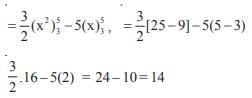
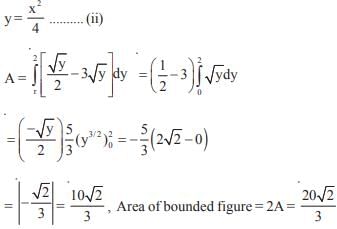
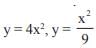 and the line y = 2 is
and the line y = 2 is