All questions of Nuclear Decay and Reactions for Grade 9 Exam
Which of the following isotopes is the most likely X in the reaction  where n denotes a free neutron?
where n denotes a free neutron?- a)
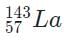
- b)
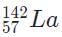
- c)
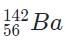
- d)
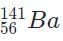
Correct answer is option 'D'. Can you explain this answer?
Which of the following isotopes is the most likely X in the reaction  where n denotes a free neutron?
where n denotes a free neutron?
 where n denotes a free neutron?
where n denotes a free neutron?a)
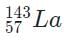
b)
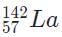
c)
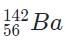
d)
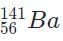
|
|
Ayesha Joshi answered |
All nuclear reactions obey conservation of mass
The mass of an isotope is given by its superscript
Each lone neutron has a mass of 1
The total mass of the released isotopes and neutrons must equal 236
Because 92 +141 + 3 = 236, the only valid isotope is
The mass of an isotope is given by its superscript
Each lone neutron has a mass of 1
The total mass of the released isotopes and neutrons must equal 236
Because 92 +141 + 3 = 236, the only valid isotope is

Suppose 64 atoms of a highly unstable Polonium isotope (half-life 10 seconds) are held in a closed container. Which of the following provides the best estimate of when there will no longer be any of the Polonium isotope left in the container?- a)There is always some isotope remaining
- b)70 s
- c)60 s
- d)30 s
Correct answer is option 'B'. Can you explain this answer?
Suppose 64 atoms of a highly unstable Polonium isotope (half-life 10 seconds) are held in a closed container. Which of the following provides the best estimate of when there will no longer be any of the Polonium isotope left in the container?
a)
There is always some isotope remaining
b)
70 s
c)
60 s
d)
30 s
|
|
Sofia Hall answered |
Analysis:
To answer this question, we need to understand the concept of half-life. The half-life of a radioactive substance is the time it takes for half of the initial amount of the substance to decay. In this case, the half-life of the Polonium isotope is given as 10 seconds.
Explanation:
To determine when there will no longer be any of the Polonium isotope left in the container, we can calculate the number of half-lives required for the initial 64 atoms to decay completely.
Calculations:
1. Initial number of atoms: 64
2. Half-life of Polonium isotope: 10 seconds
First half-life:
- After 10 seconds, half of the initial atoms will decay, leaving 32 atoms.
Second half-life:
- After another 10 seconds (total time = 20 seconds), half of the remaining 32 atoms will decay, leaving 16 atoms.
Third half-life:
- After another 10 seconds (total time = 30 seconds), half of the remaining 16 atoms will decay, leaving 8 atoms.
Fourth half-life:
- After another 10 seconds (total time = 40 seconds), half of the remaining 8 atoms will decay, leaving 4 atoms.
Fifth half-life:
- After another 10 seconds (total time = 50 seconds), half of the remaining 4 atoms will decay, leaving 2 atoms.
Sixth half-life:
- After another 10 seconds (total time = 60 seconds), half of the remaining 2 atoms will decay, leaving 1 atom.
Seventh half-life:
- After another 10 seconds (total time = 70 seconds), half of the remaining 1 atom will decay, leaving 0 atoms.
Conclusion:
Based on the calculations above, it can be concluded that after 70 seconds (option B), there will no longer be any of the Polonium isotope left in the container.
To answer this question, we need to understand the concept of half-life. The half-life of a radioactive substance is the time it takes for half of the initial amount of the substance to decay. In this case, the half-life of the Polonium isotope is given as 10 seconds.
Explanation:
To determine when there will no longer be any of the Polonium isotope left in the container, we can calculate the number of half-lives required for the initial 64 atoms to decay completely.
Calculations:
1. Initial number of atoms: 64
2. Half-life of Polonium isotope: 10 seconds
First half-life:
- After 10 seconds, half of the initial atoms will decay, leaving 32 atoms.
Second half-life:
- After another 10 seconds (total time = 20 seconds), half of the remaining 32 atoms will decay, leaving 16 atoms.
Third half-life:
- After another 10 seconds (total time = 30 seconds), half of the remaining 16 atoms will decay, leaving 8 atoms.
Fourth half-life:
- After another 10 seconds (total time = 40 seconds), half of the remaining 8 atoms will decay, leaving 4 atoms.
Fifth half-life:
- After another 10 seconds (total time = 50 seconds), half of the remaining 4 atoms will decay, leaving 2 atoms.
Sixth half-life:
- After another 10 seconds (total time = 60 seconds), half of the remaining 2 atoms will decay, leaving 1 atom.
Seventh half-life:
- After another 10 seconds (total time = 70 seconds), half of the remaining 1 atom will decay, leaving 0 atoms.
Conclusion:
Based on the calculations above, it can be concluded that after 70 seconds (option B), there will no longer be any of the Polonium isotope left in the container.
Suppose that, in a mass spectrometer, charged isotopes enter the device with velocities along a direction that is neither perpendicular nor parallel to the magnetic field lines. Which of the following behaviors would result?- a)The isotopes would travel in a corkscrew pattern
- b)The isotopes would travel in elliptical orbits
- c)The isotopes would commence uniform circular motion
- d)The isotopes would not be deflected
Correct answer is option 'A'. Can you explain this answer?
Suppose that, in a mass spectrometer, charged isotopes enter the device with velocities along a direction that is neither perpendicular nor parallel to the magnetic field lines. Which of the following behaviors would result?
a)
The isotopes would travel in a corkscrew pattern
b)
The isotopes would travel in elliptical orbits
c)
The isotopes would commence uniform circular motion
d)
The isotopes would not be deflected
|
|
William Hernandez answered |
Understanding the Behavior of Charged Isotopes in a Magnetic Field
When charged isotopes enter a magnetic field, their behavior is influenced by both their velocity and the orientation of their motion relative to the magnetic field lines. In this scenario, where the isotopes enter the magnetic field at an angle that is neither perpendicular nor parallel, the following occurs:
Corkscrew Motion
- The isotopes experience a force due to the magnetic field, known as the Lorentz force, which acts perpendicular to both the velocity of the isotopes and the direction of the magnetic field.
- This results in a centripetal force that causes the isotopes to move in a circular path while simultaneously continuing to advance in the direction of their initial velocity.
Components of Motion
- The isotopes have two components of motion:
- Circular Motion: The isotopes curve around the magnetic field lines due to the perpendicular component of their velocity. This creates a circular path.
- Linear Motion: The isotopes continue to move forward along the direction of their initial velocity.
Resulting Path
- The combination of these two motions results in a helical or corkscrew pattern:
- As the isotopes rotate around the magnetic field lines, they also advance forward, creating a spiral trajectory.
Conclusion
- Therefore, when charged isotopes enter a magnetic field at an angle, they do not follow simple circular or elliptical paths but instead exhibit a corkscrew motion. This behavior is a direct consequence of the interplay between their velocity and the magnetic field orientation, leading to the conclusion that option 'A' is indeed correct.
When charged isotopes enter a magnetic field, their behavior is influenced by both their velocity and the orientation of their motion relative to the magnetic field lines. In this scenario, where the isotopes enter the magnetic field at an angle that is neither perpendicular nor parallel, the following occurs:
Corkscrew Motion
- The isotopes experience a force due to the magnetic field, known as the Lorentz force, which acts perpendicular to both the velocity of the isotopes and the direction of the magnetic field.
- This results in a centripetal force that causes the isotopes to move in a circular path while simultaneously continuing to advance in the direction of their initial velocity.
Components of Motion
- The isotopes have two components of motion:
- Circular Motion: The isotopes curve around the magnetic field lines due to the perpendicular component of their velocity. This creates a circular path.
- Linear Motion: The isotopes continue to move forward along the direction of their initial velocity.
Resulting Path
- The combination of these two motions results in a helical or corkscrew pattern:
- As the isotopes rotate around the magnetic field lines, they also advance forward, creating a spiral trajectory.
Conclusion
- Therefore, when charged isotopes enter a magnetic field at an angle, they do not follow simple circular or elliptical paths but instead exhibit a corkscrew motion. This behavior is a direct consequence of the interplay between their velocity and the magnetic field orientation, leading to the conclusion that option 'A' is indeed correct.
When a nucleus undergoes ordinary fission into two daughter nuclei, what happens to the binding energy of the parent nucleus?- a)It is fully transferred to the binding energy of the daughter nuclei.
- b)It partly becomes the kinetic energy of the daughter nuclei
- c)It is released as a high-energy photon
- d)It is transferred into the excited energy levels of the daughter nuclei
Correct answer is option 'B'. Can you explain this answer?
When a nucleus undergoes ordinary fission into two daughter nuclei, what happens to the binding energy of the parent nucleus?
a)
It is fully transferred to the binding energy of the daughter nuclei.
b)
It partly becomes the kinetic energy of the daughter nuclei
c)
It is released as a high-energy photon
d)
It is transferred into the excited energy levels of the daughter nuclei
|
|
Ayesha Joshi answered |
- In ordinary fission, gamma ray photons are not emitted by a decaying nucleus.
- The reason that fission is energetically favorable is that the sum of the binding energies of the daughter nuclei is less than that of the parent nuclei.
- During ordinary fission, the electronic energy levels do not strongly play a role in the decay process.
- In ordinary fission, the binding energy of the parent nucleus is transformed into the kinetic energy of the daughter nuclei.
Suppose that natural nitrogen is found in 70% abundance as the isotope 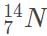 and the rest of the time as
and the rest of the time as 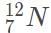 . In this scenario, which of the following would give the atomic mass of this element?
. In this scenario, which of the following would give the atomic mass of this element?- a)12.6 amu
- b)13.4 amu
- c)13.0 amu
- d)14.0 amu
Correct answer is option 'B'. Can you explain this answer?
Suppose that natural nitrogen is found in 70% abundance as the isotope 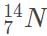 and the rest of the time as
and the rest of the time as 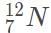 . In this scenario, which of the following would give the atomic mass of this element?
. In this scenario, which of the following would give the atomic mass of this element?
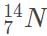 and the rest of the time as
and the rest of the time as 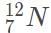 . In this scenario, which of the following would give the atomic mass of this element?
. In this scenario, which of the following would give the atomic mass of this element?a)
12.6 amu
b)
13.4 amu
c)
13.0 amu
d)
14.0 amu
|
|
Ayesha Joshi answered |
The mass of each isotope is given by its superscript.
The atomic mass of an element is the average of all isotopes of an element, weighted by their natural abundance.
Because nitrogen-14 is much more common than nitrogen-12, the average atomic mass of the element must be closer to that of nitrogen-14 than of nitrogen-12.
The atomic mass is thus .7 × 14 + .3 ∗ 12 = 13.4 amu.
The atomic mass of an element is the average of all isotopes of an element, weighted by their natural abundance.
Because nitrogen-14 is much more common than nitrogen-12, the average atomic mass of the element must be closer to that of nitrogen-14 than of nitrogen-12.
The atomic mass is thus .7 × 14 + .3 ∗ 12 = 13.4 amu.
If there is 10 kg of a radioactive isotope with a decay rate of 0.1 1/s, how much of the isotope will be left in 30 s?- a)9.0 kg
- b)0.5 kg
- c)5.0 kg
- d)0 kg
Correct answer is option 'B'. Can you explain this answer?
If there is 10 kg of a radioactive isotope with a decay rate of 0.1 1/s, how much of the isotope will be left in 30 s?
a)
9.0 kg
b)
0.5 kg
c)
5.0 kg
d)
0 kg
|
|
Ayesha Joshi answered |
Several answer choices can be eliminated by considering the properties of the exponential function. The inverse of the decay rate is 10 s, which means that the amount should decrease by several factors of e in 30 s
First write the exponential decay equation, N(t) = N0e −λt and identify that N0 = 10 Kg and λ = 0.1 1/s
Inserting t = 30 s we find that N = 10 × e-3 ≈ .5 kg
Which of the following will be a decay product when 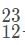 M, g undergoes beta minus decay?
M, g undergoes beta minus decay?- a)
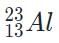
- b)
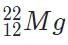
- c)
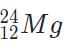
- d)
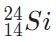
Correct answer is option 'A'. Can you explain this answer?
Which of the following will be a decay product when 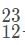 M, g undergoes beta minus decay?
M, g undergoes beta minus decay?
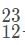 M, g undergoes beta minus decay?
M, g undergoes beta minus decay?a)
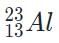
b)
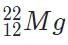
c)
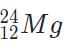
d)
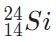
|
|
Ayesha Joshi answered |
Beta minus decay results in the release of an electron
Beta minus decay converts a neutron into a proton
The atomic number, and thus the identity of the element, increases by one during beta minus decay
The beta decay product of M, g is thus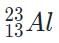 .
.
Beta minus decay converts a neutron into a proton
The atomic number, and thus the identity of the element, increases by one during beta minus decay
The beta decay product of M, g is thus
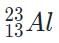 .
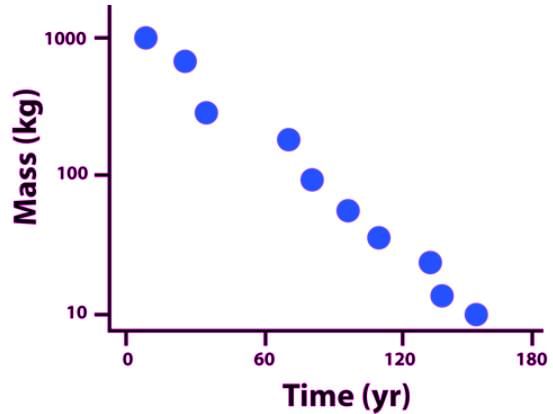
.The amount of mass of a highly unstable isotope versus time in shown in the accompanying plot. Which of the following is the best estimate of the amount of the half-life of the isotope?
- a)60 yr
- b)1 yr
- c)20 yr
- d)150 yr
Correct answer is option 'C'. Can you explain this answer?
The amount of mass of a highly unstable isotope versus time in shown in the accompanying plot. Which of the following is the best estimate of the amount of the half-life of the isotope?


a)
60 yr
b)
1 yr
c)
20 yr
d)
150 yr

|
Orion Classes answered |
Note that the semilog axis is given in base ten logarithm, and not base 1/2
You can solve this problem by approximating, without using the change-of-basis formula for logarithms
Looking carefully at the y-axis, It’s apparent that the 1000 kg mass drops down to 500 kg in way less than 60 years, but not quite as abruptly as 1 yr.
The half-life of the isotope is 20 years.
You can solve this problem by approximating, without using the change-of-basis formula for logarithms
Looking carefully at the y-axis, It’s apparent that the 1000 kg mass drops down to 500 kg in way less than 60 years, but not quite as abruptly as 1 yr.
The half-life of the isotope is 20 years.
Which of the following decay processes results in the largest change in mass of a nucleus?- a)Gamma decay
- b)Alpha decay
- c)Neutron emission
- d)Beta decay
Correct answer is option 'B'. Can you explain this answer?
Which of the following decay processes results in the largest change in mass of a nucleus?
a)
Gamma decay
b)
Alpha decay
c)
Neutron emission
d)
Beta decay
|
|
Ayesha Joshi answered |
Recall what types of particles are released during each process: Alpha decay releases helium nuclei, beta decay releases electrons, gamma decay releases photons, and neutron decay releases neutrons.
An alpha particle or helium nucleus consists of 2 neutrons and 2 protons, for a total mass of 4 atomic mass units.
Alpha decay results in the largest mass change for a decaying nucleus
Which of the following pairs of charged isotopes would be impossible to distinguish using a standard mass spectrometer?- a)

- b)
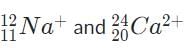
- c)

- d)
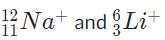
Correct answer is option 'B'. Can you explain this answer?
Which of the following pairs of charged isotopes would be impossible to distinguish using a standard mass spectrometer?
a)

b)
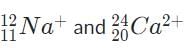
c)

d)
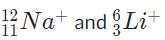
|
|
Ayesha Joshi answered |
In the standard derivation of the mass spectrometer, the centripetal acceleration due to the Lorentz force results in an equation for the radius of deflection, r = mv/qB, where q and m are the charge and mass of the isotope, respectively. B is the deflecting field, which is the same for all isotopes. v is the velocity with which the particles enter the magnetic field region.
The voltage that accelerates electrons to velocity v before entering the field region is set by energy conservation, q/V = 1/2 mv2 where V is a voltage set by the machine that remains constant for all ions. This means that the entering velocity v depends only on the ratio q/m, for the isotopes.
The pair of isotopes, 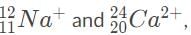 are thus impossible to distinguish because they have the same charge-to-mass ratio and thus are deflected the same amount by the magnetic field.
are thus impossible to distinguish because they have the same charge-to-mass ratio and thus are deflected the same amount by the magnetic field.
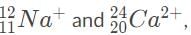 are thus impossible to distinguish because they have the same charge-to-mass ratio and thus are deflected the same amount by the magnetic field.
are thus impossible to distinguish because they have the same charge-to-mass ratio and thus are deflected the same amount by the magnetic field.Chapter doubts & questions for Nuclear Decay and Reactions - Physics 2025 is part of Grade 9 exam preparation. The chapters have been prepared according to the Grade 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Nuclear Decay and Reactions - Physics in English & Hindi are available as part of Grade 9 exam.
Download more important topics, notes, lectures and mock test series for Grade 9 Exam by signing up for free.
Physics
307 videos|482 docs|202 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









