All Exams >
NEET >
1 Year Dropper Course for NEET >
All Questions
All questions of Purification and Characterisation of Organic Compounds for NEET Exam
The best method for the separation of naphthalene and benzoic acid from their mixture is- a)chromatography
- b)crystallisation
- c)distillation
- d)sublimation
Correct answer is option 'D'. Can you explain this answer?
The best method for the separation of naphthalene and benzoic acid from their mixture is
a)
chromatography
b)
crystallisation
c)
distillation
d)
sublimation

|
EduRev NEET answered |
The best method for the separation of naphthalene and benzoic acid from their mixture is sublimation because it is applicable for those organic compounds which pass directly from solid to vapour state on heating and vice versa on cooling.
In these compounds naphthalene is volatile and benzoic acid is non-volatile due to the formation of dimer via hydrogen bonding (intermolecular).
In these compounds naphthalene is volatile and benzoic acid is non-volatile due to the formation of dimer via hydrogen bonding (intermolecular).
A is a lighter phenol and B is an aromatic carboxylic acid. Separation of a mixture of A and B can be carried out easily by using a solution of- a)sodium hydroxide
- b)sodium sulphate
- c)calcium chloride
- d)sodium bicarbonate
Correct answer is option 'D'. Can you explain this answer?
A is a lighter phenol and B is an aromatic carboxylic acid. Separation of a mixture of A and B can be carried out easily by using a solution of
a)
sodium hydroxide
b)
sodium sulphate
c)
calcium chloride
d)
sodium bicarbonate

|
Infinity Academy answered |
Carboxylic acids are soluble in sodium bicarbonate but phenol are not dissolve in it, so they are separated because carboxylic acid react with NaHCO3 and form sodium carboxylate.
R — COOH + NaHCO3 → R — COO−Na+ + H2CO3
R — COOH + NaHCO3 → R — COO−Na+ + H2CO3
Camphor is often used in molecular mass determination because- a)it is readily available
- b)it has a very high cryoscopic constant
- c)it is volatile
- d)it is solvent for organic substances
Correct answer is option 'C'. Can you explain this answer?
Camphor is often used in molecular mass determination because
a)
it is readily available
b)
it has a very high cryoscopic constant
c)
it is volatile
d)
it is solvent for organic substances

|
Top Rankers answered |
Camphor is used in molecular mass determination due to its volatile nature.
The method is called Rast’s camphor method. Camphor acts as a solid solvent which is volatile, hence can be removed easily.
The method is called Rast’s camphor method. Camphor acts as a solid solvent which is volatile, hence can be removed easily.
In steam distillation of toluene, the pressure of toluene in vapour is- a)equal to the pressure of barometer
- b)less than the pressure of barometer
- c)equal to vapour pressure of toluene in simple distillation
- d)more than vapour pressure of toluene in simple distillation
Correct answer is option 'B'. Can you explain this answer?
In steam distillation of toluene, the pressure of toluene in vapour is
a)
equal to the pressure of barometer
b)
less than the pressure of barometer
c)
equal to vapour pressure of toluene in simple distillation
d)
more than vapour pressure of toluene in simple distillation

|
Lead Academy answered |
In steam distillation of toluene, the pressure of toluene in vapour is less than pressure of barometer, because it is carried out when a solid or liquid is insoluble in water and is volatile with steam but the impurities are non-volatile.
Which of the statements is not true?- a)On passing H2S through acidified K2Cr2O7 solution, a milky colour is observed
- b)Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis
- c)K2Cr2O7 solution in acidic medium is orange
- d)K2Cr2O7 solution becomes yellow on increasing the pH beyond 7
Correct answer is option 'B'. Can you explain this answer?
Which of the statements is not true?
a)
On passing H2S through acidified K2Cr2O7 solution, a milky colour is observed
b)
Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis
c)
K2Cr2O7 solution in acidic medium is orange
d)
K2Cr2O7 solution becomes yellow on increasing the pH beyond 7

|
Ciel Knowledge answered |
Being hygroscopic, sodium dichromate, Na2Cr2O7 cannot be used in volumetric analysis.
All other given statements are true.
All other given statements are true.
The most suitable method of separation of 1:1 mixture of ortho and para-nitrophenols is- a)sublimation
- b)chromatography
- c)crystallisation
- d)steam distillation
Correct answer is option 'D'. Can you explain this answer?
The most suitable method of separation of 1:1 mixture of ortho and para-nitrophenols is
a)
sublimation
b)
chromatography
c)
crystallisation
d)
steam distillation

|
Stepway Academy answered |
Steam distillation is used to purify the substances which
(i) are volatile in steam but are immiscible with water.
(ii) possess sufficiently high vapour pressure at the boiling point of water.
(iii) contain non- volatile impurities.
The process of steam distillation can also be used to separate a mixture of two organic compounds one of which is steam volatile while the other is not.
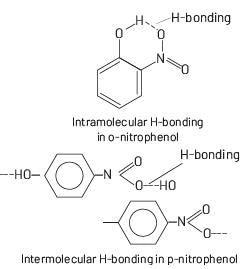
In ortho and para-nitrophenol, ortho-nitrophenol has intramolecular H-bonding. So, it has lower boiling point.
Intermolecular H-Bonding more strong then intramolecular H-bonding. Whereas para-nitrophenol has intermolecular H-bonding. So, it has higher boiling point.
Due to difference in boiling points ortho and para-nitrophenol can be separated from each other by distillation.
(i) are volatile in steam but are immiscible with water.
(ii) possess sufficiently high vapour pressure at the boiling point of water.
(iii) contain non- volatile impurities.
The process of steam distillation can also be used to separate a mixture of two organic compounds one of which is steam volatile while the other is not.
In ortho and para-nitrophenol, ortho-nitrophenol has intramolecular H-bonding. So, it has lower boiling point.
Intermolecular H-Bonding more strong then intramolecular H-bonding. Whereas para-nitrophenol has intermolecular H-bonding. So, it has higher boiling point.
Due to difference in boiling points ortho and para-nitrophenol can be separated from each other by distillation.

Which of the following techniques is most suitable for purification of cyclohexanone from a mixture containing benzoic acid, isoamyl alcohol, cyclohexane and cyclohexanone?- a)Crystallisation
- b)IR spectroscopy
- c)Sublimation
- d)Evaporation
Correct answer is option 'B'. Can you explain this answer?
Which of the following techniques is most suitable for purification of cyclohexanone from a mixture containing benzoic acid, isoamyl alcohol, cyclohexane and cyclohexanone?
a)
Crystallisation
b)
IR spectroscopy
c)
Sublimation
d)
Evaporation

|
Lead Academy answered |
IR spectroscopy is used for the purification of cyclohexanone from a mixture of benzoic acid, isoamyl alcohol, cyclohexane and cyclohexanone because in IR spectroscopy each functional group appears at a certain peak. IR spectroscopy exploits the fact that molecules absorb specific frequencies that are characteristic of their structure.
Prussian blue is formed when- a)ferrous sulphate reacts with FeCl3
- b)ferric sulphate reacts with Na4[Fe(CN)6]
- c)ferrous ammonium sulphate reacts with FeCl3
- d)ammonium sulphate reacts with FeCl3
Correct answer is option 'B'. Can you explain this answer?
Prussian blue is formed when
a)
ferrous sulphate reacts with FeCl3
b)
ferric sulphate reacts with Na4[Fe(CN)6]
c)
ferrous ammonium sulphate reacts with FeCl3
d)
ammonium sulphate reacts with FeCl3

|
Bs Academy answered |
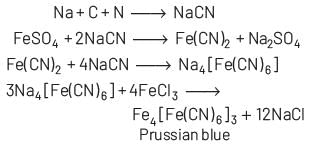
When the sodium fusion extract is added with FeCl3 and then the resulting solution is acidified with dilute hydrochloric acid, the appearance of Prussian blue colouration confirms the presence of nitrogen in the organic compound.

Lassaigne’s test for the detection of nitrogen fails in- a)NH2CONHNH2⋅HCl
- b)NH2NH2⋅HCl
- c)NH2CONH2
- d)C6H5NHNH2⋅HCl
Correct answer is option 'B'. Can you explain this answer?
Lassaigne’s test for the detection of nitrogen fails in
a)
NH2CONHNH2⋅HCl
b)
NH2NH2⋅HCl
c)
NH2CONH2
d)
C6H5NHNH2⋅HCl

|
Ambition Institute answered |
Lassaigne’s test is given by only those compounds which contain both carbon and nitrogen. When compounds containing C and N heated with sodium, then it form NaCN which is easily detected byFeCl3.
Or
Some compounds live hydrazine (NH2⋅ NH2) although contain nitrogen but they do not respond Lassaigne’s test because they do not have any carbon and hence, NaCN is not formed.
Or
Some compounds live hydrazine (NH2⋅ NH2) although contain nitrogen but they do not respond Lassaigne’s test because they do not have any carbon and hence, NaCN is not formed.
The Lassaigne’s extract is boiled with conc. HNO3 while testing for halogens. By doing so it- a)helps in the precipitation of AgCl
- b)increases the solubility product of AgCl
- c)increases the concentration of NO3–ions
- d)decomposes Na2S and NaCN, if formed
Correct answer is option 'D'. Can you explain this answer?
The Lassaigne’s extract is boiled with conc. HNO3 while testing for halogens. By doing so it
a)
helps in the precipitation of AgCl
b)
increases the solubility product of AgCl
c)
increases the concentration of NO3–ions
d)
decomposes Na2S and NaCN, if formed

|
Bs Academy answered |
Na2S and NaCN, if present in the extract, will be decomposed to H2S and HCN by HNO3.
NaCN + HNO3 → NaNO3 + HCN
Na2S + 2HNO3 → 2NaNO3 + H2S
These will escape from the solution and will not interfere with the test for halogens.
NaCN + HNO3 → NaNO3 + HCN
Na2S + 2HNO3 → 2NaNO3 + H2S
These will escape from the solution and will not interfere with the test for halogens.
Chapter doubts & questions for Purification and Characterisation of Organic Compounds - 1 Year Dropper Course for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Purification and Characterisation of Organic Compounds - 1 Year Dropper Course for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









