All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Titration (GC) for MCAT Exam
1 M of a weak acid H Z with Kα = 10e - 8 equilibrates in water according to the equation H Z + H2O → H3O+ + Z-. What is the pH of the solution at equilibrium?- a)7
- b)6
- c)4
- d)2
Correct answer is option 'C'. Can you explain this answer?
1 M of a weak acid H Z with Kα = 10e - 8 equilibrates in water according to the equation H Z + H2O → H3O+ + Z-. What is the pH of the solution at equilibrium?
a)
7
b)
6
c)
4
d)
2
|
|
Ayesha Joshi answered |
Unlike a strong acid, a weak acid does not completely neutralize; instead an Initial-Change-Equilibrium (ICE) table may be constructed for this problem.
For every unit x of H Z that reacts, a unit x of both H3O+ and Z- is created due to the balanced reaction equation.
The expression for the dissociation constant of an acid in water is 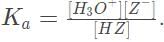 Substituting our values into this expression results in the equation 10e - 8 =
Substituting our values into this expression results in the equation 10e - 8 = 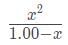
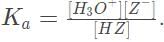 Substituting our values into this expression results in the equation 10e - 8 =
Substituting our values into this expression results in the equation 10e - 8 = 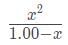
Because H Z is a weak acid, approximate 1.00 - x ≈ 1.00 and solve the resulting equation x2 = 10e - 8 ⇒ 10e - 4, where x is the equilibrium concentration of acid The pH is thus pH = -log(10e - 4) = 4
Titration curves exhibit an asymptote at very large volumes of added titrant. Which of the following experimental parameters determines the location of this asymptote?- a)The pH of the initial solution
- b)The slope of the curve on the titration curve
- c)The Ka of the initial solution
- d)The pH of the titrant
Correct answer is option 'D'. Can you explain this answer?
Titration curves exhibit an asymptote at very large volumes of added titrant. Which of the following experimental parameters determines the location of this asymptote?
a)
The pH of the initial solution
b)
The slope of the curve on the titration curve
c)
The Ka of the initial solution
d)
The pH of the titrant
|
|
Ayesha Joshi answered |
Because this question asks about the limiting case of an excess of titrant, you can pick any titrant volume that is in excess of that needed to obtain the equivalence point. Not knowing anything about the species being titrated, a safe bet is to use the case where an infinite amount of titrant has been added to the solution.
In the limit of an infinitely amount of titrant has been added to the solution, the pH should become just the pH of the titrant---the original solution gets diluted away.
We thus expect the asymptotic pH of a titration curve to approach that of the titrant.
Which of the following describes the “buffer” region in a titration curve?- a)The flat portion of the curve long after the equivalence point has been reached
- b)The flat portion of the curve just before the equivalence point
- c)The steep portion of the curve immediately after the equivalence point
- d)The concave portion of the curve immediately after the addition of titrant
Correct answer is option 'B'. Can you explain this answer?
Which of the following describes the “buffer” region in a titration curve?
a)
The flat portion of the curve long after the equivalence point has been reached
b)
The flat portion of the curve just before the equivalence point
c)
The steep portion of the curve immediately after the equivalence point
d)
The concave portion of the curve immediately after the addition of titrant
|
|
Ava Brown answered |
Which of the following options are you referring to? Please provide more context.
33 mL of 3 M Hydrochloric acid is titrated with sodium hydroxide to form water and sodium chloride. How many mmols of sodium hydroxide are consumed in this reaction?- a)3 mmols
- b)10 mmols
- c)33 mmols
- d)100 mmols
Correct answer is option 'D'. Can you explain this answer?
33 mL of 3 M Hydrochloric acid is titrated with sodium hydroxide to form water and sodium chloride. How many mmols of sodium hydroxide are consumed in this reaction?
a)
3 mmols
b)
10 mmols
c)
33 mmols
d)
100 mmols
|
|
Dylan Perez answered |
To determine the number of millimoles (mmols) of sodium hydroxide (NaOH) consumed in the reaction, we need to use the stoichiometry of the balanced chemical equation.
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and sodium hydroxide is:
HCl + NaOH → H2O + NaCl
From the equation, we can see that 1 mole of HCl reacts with 1 mole of NaOH to produce 1 mole of water and 1 mole of NaCl.
Given that the volume of HCl is 33 mL and the concentration is 3 M (moles per liter), we can calculate the number of moles of HCl:
Moles of HCl = concentration (M) × volume (L)
Converting the volume from milliliters to liters:
Volume of HCl = 33 mL = 33/1000 L = 0.033 L
Moles of HCl = 3 M × 0.033 L = 0.099 moles
Since the stoichiometry of the reaction is 1:1 between HCl and NaOH, the number of moles of NaOH consumed will also be 0.099 moles.
To convert moles to millimoles, we multiply by 1000:
Millimoles of NaOH = 0.099 moles × 1000 = 99 mmols
Therefore, the correct answer is option 'D', 100 mmols.
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and sodium hydroxide is:
HCl + NaOH → H2O + NaCl
From the equation, we can see that 1 mole of HCl reacts with 1 mole of NaOH to produce 1 mole of water and 1 mole of NaCl.
Given that the volume of HCl is 33 mL and the concentration is 3 M (moles per liter), we can calculate the number of moles of HCl:
Moles of HCl = concentration (M) × volume (L)
Converting the volume from milliliters to liters:
Volume of HCl = 33 mL = 33/1000 L = 0.033 L
Moles of HCl = 3 M × 0.033 L = 0.099 moles
Since the stoichiometry of the reaction is 1:1 between HCl and NaOH, the number of moles of NaOH consumed will also be 0.099 moles.
To convert moles to millimoles, we multiply by 1000:
Millimoles of NaOH = 0.099 moles × 1000 = 99 mmols
Therefore, the correct answer is option 'D', 100 mmols.
10 mL of 0.5 M calcium hydroxide is required to titrate 50 mL hydrochloric acid. Which of the following gives the initial concentration of the acid?- a)1/5 M
- b)1/10 M
- c)5 M
- d)10 M
Correct answer is option 'A'. Can you explain this answer?
10 mL of 0.5 M calcium hydroxide is required to titrate 50 mL hydrochloric acid. Which of the following gives the initial concentration of the acid?
a)
1/5 M
b)
1/10 M
c)
5 M
d)
10 M
|
|
Ayesha Joshi answered |
The balanced reaction is
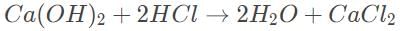
First find the number of mols that reacted, 0.5 x 10 = 5 mmol. According to the balanced reaction, this requires 10mmol of HCl
Find the concentration of the acid using the definition of molarity, 10/50 = 1/5 M
Which of the following criteria accurately describes the primary difference between a strong versus weak acid?- a)Negative versus positive pH
- b)Presence versus absence of a halogen ion in the chemical structure
- c)Proton donation versus electron acceptance
- d)Complete versus partial ionization in solution
Correct answer is option 'D'. Can you explain this answer?
Which of the following criteria accurately describes the primary difference between a strong versus weak acid?
a)
Negative versus positive pH
b)
Presence versus absence of a halogen ion in the chemical structure
c)
Proton donation versus electron acceptance
d)
Complete versus partial ionization in solution
|
|
Ayesha Joshi answered |
While an acid with a pH less than 0 is likely a strong acid, many strong acids can have pH greater than zero depending on their concentration
Proton donation versus electron acceptance differentiate Bronsted-Lowry and Lewis acids, but not their relative strengths
Complete versus partial ionization in solution is the primary determinant of whether an acid is considered weak.
Which of the following describes the equivalence point on a graph of pH versus the amount of titrant added to a solution?- a)The point where the magnitude of the slope of the curve is greatest
- b)The point on the curve with the lowest pH
- c)The point on the curve with highest pH
- d)The point where the magnitude of the slope of the curve is least
Correct answer is option 'A'. Can you explain this answer?
Which of the following describes the equivalence point on a graph of pH versus the amount of titrant added to a solution?
a)
The point where the magnitude of the slope of the curve is greatest
b)
The point on the curve with the lowest pH
c)
The point on the curve with highest pH
d)
The point where the magnitude of the slope of the curve is least
|
|
Ayesha Joshi answered |
During a titration, as titrant is added the pH gradually changes from the pH of the initial solution to being the pH of the titrant.
The pH takes on maximal values only at maximal values on the horizontal (volume) axis
The “crossover” between the portion of the graph where the solution has pH closer to the initial solution, and the portion in which the titrant is in excess, is known as the equivalence point
The magnitude of the slope of the graph is greatest at the equivalence point.
Which of the following pH indicator ranges would be the most useful for the titration of a weak base with a strong acid?- a)4 - 6
- b)7 - 8
- c)5 - 8
- d)8 - 10
Correct answer is option 'A'. Can you explain this answer?
Which of the following pH indicator ranges would be the most useful for the titration of a weak base with a strong acid?
a)
4 - 6
b)
7 - 8
c)
5 - 8
d)
8 - 10
|
|
Ayesha Joshi answered |
The equivalence point of the titration of a weak base by a strong acid will occur at a pH less than 7
A good indicator will be able to indicate the equivalence point right as it occurs, making a narrow range preferable to a large range like 5 - 8
The best indicator has a pH in the range 4 - 6
50 mL of 0.5 M barium hydroxide is required to fully titrate a 100 mL solution of sulfuric acid. What is the initial concentration of the acid?- a)100 M
- b).25 M
- c)50 M
- d).5 M
Correct answer is option 'B'. Can you explain this answer?
50 mL of 0.5 M barium hydroxide is required to fully titrate a 100 mL solution of sulfuric acid. What is the initial concentration of the acid?
a)
100 M
b)
.25 M
c)
50 M
d)
.5 M
|
|
Ayesha Joshi answered |
The formulae for barium hydroxide and sulfuric acid are
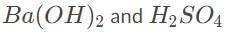
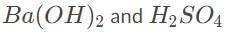
The balanced reaction is
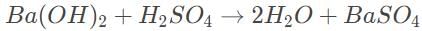
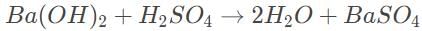
Because the stoichiometric ratio of the reactants is 1 : 1, we can use the shortcut formula MbVb = MαVα
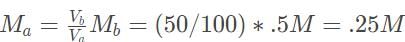
In a titration of a weak acid with a strong base, what is the pH of the solution at the equivalence point?- a)> 7
- b)< 7
- c)7
- d)0
Correct answer is option 'A'. Can you explain this answer?
In a titration of a weak acid with a strong base, what is the pH of the solution at the equivalence point?
a)
> 7
b)
< 7
c)
7
d)
0
|
|
Ayesha Joshi answered |
In a weak-strong titration, the equivalence point is shifted from neutralization towards the pH of the strong reagent (a base here)
The equivalence point occurs when the mols of acid added stoichiometrically balances the moles of base added.
The pH of a weak acid, strong base titration is greater than 7 at the equivalence point.
Chapter doubts & questions for Titration (GC) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Titration (GC) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









