All Exams >
NEET >
Chemistry 31 Years NEET Chapterwise Solved Papers >
All Questions
All questions of Redox Reactions for NEET Exam
The loss of electron is termed as [1995]- a)oxidation
- b)reduction
- c)combustion
- d)neutralization
Correct answer is option 'A'. Can you explain this answer?
The loss of electron is termed as [1995]
a)
oxidation
b)
reduction
c)
combustion
d)
neutralization

|
Mahesh Saini answered |
Losing of electron is called oxidation,
Oxidation numbers of P in PO43– , of S in SO42– and that of Cr in Cr2O72– are respectively [2009]- a)+ 3, + 6 and + 5
- b)+ 5, + 3 and + 6
- c)– 3, + 6 and + 6
- d)+ 5, + 6 and + 6
Correct answer is option 'D'. Can you explain this answer?
Oxidation numbers of P in PO43– , of S in SO42– and that of Cr in Cr2O72– are respectively [2009]
a)
+ 3, + 6 and + 5
b)
+ 5, + 3 and + 6
c)
– 3, + 6 and + 6
d)
+ 5, + 6 and + 6
|
|
Hansa Sharma answered |
(i) Sum of oxidation states of all atoms = charge of ion.
(ii) oxidation number of oxygen = -2
Let the oxidation state of P in PO43- is x.
PO43-
x + 4 (-2) = - 3
x-8 = - 3
x = +5
Let the oxidation state of S in SO42- is y
y + 4(-2) = -2
y-8 = - 2
y = +6
Let the oxidation state of Cr in Cr2O72- is z.
2 x z+7(-2) = -2
2z-14 = - 2
z=+6
Hence, oxidation state of P, S and Cr are +5, +6 and +6
(ii) oxidation number of oxygen = -2
Let the oxidation state of P in PO43- is x.
PO43-
x + 4 (-2) = - 3
x-8 = - 3
x = +5
Let the oxidation state of S in SO42- is y
y + 4(-2) = -2
y-8 = - 2
y = +6
Let the oxidation state of Cr in Cr2O72- is z.
2 x z+7(-2) = -2
2z-14 = - 2
z=+6
Hence, oxidation state of P, S and Cr are +5, +6 and +6
Which of the following involves a redox reaction?- a)Reaction of H2SO4 with NaOH
- b)Production of ozone from oxygen in the atmosphere by lightning
- c)Production of nitrogen oxides from nitrogen and oxygen in the atmosphere by lightning
- d)Evaporation of water
Correct answer is option 'C'. Can you explain this answer?
Which of the following involves a redox reaction?
a)
Reaction of H2SO4 with NaOH
b)
Production of ozone from oxygen in the atmosphere by lightning
c)
Production of nitrogen oxides from nitrogen and oxygen in the atmosphere by lightning
d)
Evaporation of water
|
|
Rocky Handsome answered |
•The 1st reaction is acid base reaction which does not involve either oxidation or reduction.
•O3 formation from O2 does not involve either oxidation or reduction.
•Nitrogen oxides from N2 and O2 involves oxidation of nitrogen and reduction of oxygen.
For example, N2 + O2---> NO2.
• Evaporation of H2O is a physical change and is not a chemical change.
Hence answer is C.
•O3 formation from O2 does not involve either oxidation or reduction.
•Nitrogen oxides from N2 and O2 involves oxidation of nitrogen and reduction of oxygen.
For example, N2 + O2---> NO2.
• Evaporation of H2O is a physical change and is not a chemical change.
Hence answer is C.
In which of the following reactions, there is no change in valency ? [1994]- a)4 KClO3 —→ 3KClO4 + KCl
- b)SO2 + 2H2S —→ 2H2O + 3S
- c)BaO2 + H2SO4 —→ BaSO4 + H2O2
- d)3 BaO + O2 —→ 2 BaO2.
Correct answer is option 'C'. Can you explain this answer?
In which of the following reactions, there is no change in valency ? [1994]
a)
4 KClO3 —→ 3KClO4 + KCl
b)
SO2 + 2H2S —→ 2H2O + 3S
c)
BaO2 + H2SO4 —→ BaSO4 + H2O2
d)
3 BaO + O2 —→ 2 BaO2.

|
Maheshwar Saini answered |
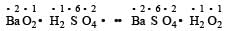
In this reaction, none of the elements undergoes a change in oxidation number or valency.
The oxidation number of chromium in potassium dichromate is [1995]- a)+ 6
- b)– 5
- c)– 2
- d)+ 2
Correct answer is option 'A'. Can you explain this answer?
The oxidation number of chromium in potassium dichromate is [1995]
a)
+ 6
b)
– 5
c)
– 2
d)
+ 2

|
Ayush Sengupta answered |
Let x = oxidation no. of Cr in K2Cr2O7.
∴ (2 × 1) + (2 × x) + 7 (– 2) = 0
or 2 + 2x – 14 = 0 or x = + 6.
∴ (2 × 1) + (2 × x) + 7 (– 2) = 0
or 2 + 2x – 14 = 0 or x = + 6.
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes maximum change in the oxidation number ? [2012]- a)S
- b)H
- c)Cl
- d)C
Correct answer is option 'C'. Can you explain this answer?
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes maximum change in the oxidation number ? [2012]
a)
S
b)
H
c)
Cl
d)
C

|
Ayush Sengupta answered |
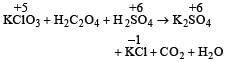
i.e. maximum change in oxidation number is observed in Cl (+5 to –1).
Phosphorus has the oxidation state of + 3 in- a)Phosphorous acid [1994]
- b)Orthophosphoric acid
- c)Hypophosphorous acid
- d)Metaphosphoric acid.
Correct answer is option 'A'. Can you explain this answer?
Phosphorus has the oxidation state of + 3 in
a)
Phosphorous acid [1994]
b)
Orthophosphoric acid
c)
Hypophosphorous acid
d)
Metaphosphoric acid.

|
Sushant Goyal answered |
O.N. of P in H3PO3 (phosphorous acid) 3 × 1 + x + 3 × (– 2) = 0 or x = + 3 In orthophosphoric acid (H3PO4) O.N. of P is + 5, in hypophosphorous acid (H3PO2) it is + 1 while in metaphosphoric acid (HPO3), it is + 5,
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from : [2012]- a)zero to +1 and zero to –5
- b)zero to –1 and zero to +5
- c)zero to –1 and zero to +3
- d)zero to +1 and zero to –3
Correct answer is option 'B'. Can you explain this answer?
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from : [2012]
a)
zero to +1 and zero to –5
b)
zero to –1 and zero to +5
c)
zero to –1 and zero to +3
d)
zero to +1 and zero to –3

|
Arnab Iyer answered |
On reaction with hot and concentrated alkali a mixture of chloride and chlorate is formed
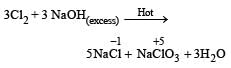
The oxidation number of phosphorus in pyrophosphoric acid is [1999]- a)+3
- b)+1
- c)+4
- d)+5
Correct answer is option 'D'. Can you explain this answer?
The oxidation number of phosphorus in pyrophosphoric acid is [1999]
a)
+3
b)
+1
c)
+4
d)
+5
|
|
Keerthana Datta answered |
The oxidation number of an element in a compound is a measure of the charge that element would have if all the bonding electrons were assigned to the more electronegative atom. Oxidation numbers are used to track the flow of electrons in chemical reactions and to determine the potential for redox reactions.
In pyrophosphoric acid (H4P2O7), there are two phosphorus atoms. To determine the oxidation number of phosphorus in this compound, we can use the following steps:
1. Assign oxidation numbers to the other elements in the compound:
- Hydrogen (H) is usually assigned an oxidation number of +1.
- Oxygen (O) is usually assigned an oxidation number of -2.
2. Determine the overall charge of the compound:
- The overall charge of pyrophosphoric acid is 0, since it is a neutral compound.
3. Apply the oxidation number rules to determine the oxidation number of phosphorus:
- The sum of all oxidation numbers in a compound must equal the overall charge of the compound.
- Since there are two phosphorus atoms in pyrophosphoric acid, let's assign the oxidation number of one of the phosphorus atoms as "x".
4. Calculate the oxidation number of phosphorus:
- The sum of the oxidation numbers of all the elements in the compound must equal zero. Therefore, we can write the equation:
(4)(+1) + 2(x) + (7)(-2) = 0
4 + 2x - 14 = 0
2x - 10 = 0
2x = 10
x = 5
Therefore, the oxidation number of phosphorus in pyrophosphoric acid is +5 or 5. Thus, the correct answer is option 'D'.
In pyrophosphoric acid (H4P2O7), there are two phosphorus atoms. To determine the oxidation number of phosphorus in this compound, we can use the following steps:
1. Assign oxidation numbers to the other elements in the compound:
- Hydrogen (H) is usually assigned an oxidation number of +1.
- Oxygen (O) is usually assigned an oxidation number of -2.
2. Determine the overall charge of the compound:
- The overall charge of pyrophosphoric acid is 0, since it is a neutral compound.
3. Apply the oxidation number rules to determine the oxidation number of phosphorus:
- The sum of all oxidation numbers in a compound must equal the overall charge of the compound.
- Since there are two phosphorus atoms in pyrophosphoric acid, let's assign the oxidation number of one of the phosphorus atoms as "x".
4. Calculate the oxidation number of phosphorus:
- The sum of the oxidation numbers of all the elements in the compound must equal zero. Therefore, we can write the equation:
(4)(+1) + 2(x) + (7)(-2) = 0
4 + 2x - 14 = 0
2x - 10 = 0
2x = 10
x = 5
Therefore, the oxidation number of phosphorus in pyrophosphoric acid is +5 or 5. Thus, the correct answer is option 'D'.
The oxidation states of sulphur in the anions SO32–, S2O42– and S2O62– follow the order [2003]- a)
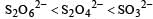
- b)
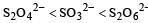
- c)
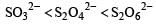
- d)
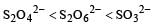
Correct answer is option 'B'. Can you explain this answer?
The oxidation states of sulphur in the anions SO32–, S2O42– and S2O62– follow the order [2003]
a)
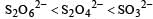
b)
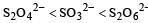
c)
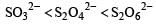
d)
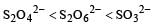
|
|
Suresh Reddy answered |
The correct answer is option B
Oxidation state of S2O42−
2(x) + 4(−2) = −2
2x = 8 − 2
2x = 6
x = 3
Oxidation state of SO32−
x + 3(−2) = −2
x = 6 − 2
x = 4
Oxidation state of S2O62−
2(x) + 6(−2) = −2
2x = 12 − 2
2x = 10
x = 5
So the oxidation state of sulphur in the anions S2O42−, S2O42− and S2O62− follows the order.S2O42− < SO32− < S2O62−.
Oxidation state of S2O42−
2(x) + 4(−2) = −2
2x = 8 − 2
2x = 6
x = 3
Oxidation state of SO32−
x + 3(−2) = −2
x = 6 − 2
x = 4
Oxidation state of S2O62−
2(x) + 6(−2) = −2
2x = 12 − 2
2x = 10
x = 5
So the oxidation state of sulphur in the anions S2O42−, S2O42− and S2O62− follows the order.S2O42− < SO32− < S2O62−.
The oxide, which cannot act as a reducing agent, is[1995]- a)NO2
- b)SO2
- c)CO2
- d)ClO2
Correct answer is option 'C'. Can you explain this answer?
The oxide, which cannot act as a reducing agent, is[1995]
a)
NO2
b)
SO2
c)
CO2
d)
ClO2

|
Shounak Nair answered |
Carbon has the maximum oxidation state of + 4, therefore carbon dioxide (CO2) cannot act as a reducing agent.
A compound contains atoms of three elements A, B and C. If the oxidation number of A is +2, B is +5, and that of C is –2, the possible formula of the compound is : [2000]- a)A2(BC3)2
- b)A3(BC4)2
- c)A3(B4C)2
- d)ABC2
Correct answer is option 'B'. Can you explain this answer?
A compound contains atoms of three elements A, B and C. If the oxidation number of A is +2, B is +5, and that of C is –2, the possible formula of the compound is : [2000]
a)
A2(BC3)2
b)
A3(BC4)2
c)
A3(B4C)2
d)
ABC2

|
Mahesh Saini answered |
Oxidation number of a compound must be 0.
Using the values for A, B and C in the four options we find that A3(BC4)2 is the answer.
Check : (+2)3 + [(+5)+4(–2)]2 = 6 + (5–8)2 = 0
Using the values for A, B and C in the four options we find that A3(BC4)2 is the answer.
Check : (+2)3 + [(+5)+4(–2)]2 = 6 + (5–8)2 = 0
The following redox reaction is balanced by which set of coefficients ? [1999]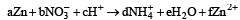
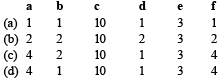
- a)a
- b)b
- c)c
- d)d
Correct answer is option ''. Can you explain this answer?
The following redox reaction is balanced by which set of coefficients ? [1999]
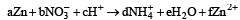
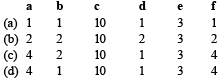
a)
a
b)
b
c)
c
d)
d

|
Shanaya Rane answered |
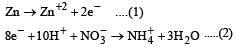
operate eq. (1) × 4 + eq. (2) × 1
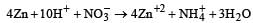
Zn gives H2 gas with H2SO4 and HCl but not with HNO3 because [2002]- a)Zn acts as an oxidising agent when it reacts with HNO3
- b)HNO3 is weaker acid than H2SO4 and HCl
- c)In electroch emical series, Zn is above hydrogen
- d)
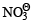 is reduced in preference to hydronium ion
is reduced in preference to hydronium ion
Correct answer is option 'D'. Can you explain this answer?
Zn gives H2 gas with H2SO4 and HCl but not with HNO3 because [2002]
a)
Zn acts as an oxidising agent when it reacts with HNO3
b)
HNO3 is weaker acid than H2SO4 and HCl
c)
In electroch emical series, Zn is above hydrogen
d)
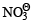 is reduced in preference to hydronium ion
is reduced in preference to hydronium ion

|
Pankaj Banerjee answered |
Zinc is or.r the top position of hydrogen in electrochemical series. So Zn displaces H2 from dilute H2SO4 and HCl with iiberation of H2.
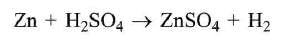
On the other hand HNO3 is an oxidising agent. Hydrogen obtained in this reaction is converted into H2O.
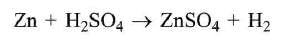
On the other hand HNO3 is an oxidising agent. Hydrogen obtained in this reaction is converted into H2O.
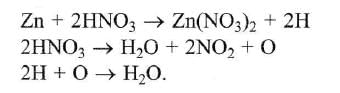
Which substan ce serves as reducing agent in the following reaction ? [1994]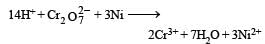
- a)H2O
- b)Ni
- c)H+
- d)
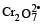
Correct answer is option 'B'. Can you explain this answer?
Which substan ce serves as reducing agent in the following reaction ? [1994]
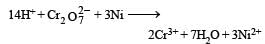
a)
H2O
b)
Ni
c)
H+
d)

|
Prashanth Dasgupta answered |
The element undergo oxidation itself and reduces others is known as reducing agent.
In this reaction O. N. of Ni Changes from 0 to + 2 and hence Ni acts as a reducing agent.
In this reaction O. N. of Ni Changes from 0 to + 2 and hence Ni acts as a reducing agent.
Standard reduction potentials of the half reactions are given below :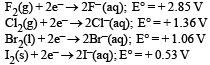 The strongest oxidising and reducing agents respectively are : [2012 M]
The strongest oxidising and reducing agents respectively are : [2012 M]- a)F2 and I–
- b)Br2 and Cl–
- c)Cl2 and Br–
- d)Cl2 and I2
Correct answer is option 'A'. Can you explain this answer?
Standard reduction potentials of the half reactions are given below :
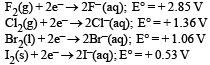
The strongest oxidising and reducing agents respectively are : [2012 M]
a)
F2 and I–
b)
Br2 and Cl–
c)
Cl2 and Br–
d)
Cl2 and I2

|
Arindam Khanna answered |
Higher the value of reduction potential higher will be the oxidising power whereas the lower the value of reduction potential higher will be the reducing power.
Chapter doubts & questions for Redox Reactions - Chemistry 31 Years NEET Chapterwise Solved Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Redox Reactions - Chemistry 31 Years NEET Chapterwise Solved Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









