All Exams >
Class 10 >
CBSE Sample Papers For Class 10 >
All Questions
All questions of CBSE Sample Question Papers for 2021-22 for Class 10 Exam
Which of the following mirror is used by a dentist to examine a small cavity in a patient’s teeth?- a)Convex mirror
- b)Plane mirror
- c)Concave mirror
- d)Any spherical mirror
Correct answer is option 'C'. Can you explain this answer?
Which of the following mirror is used by a dentist to examine a small cavity in a patient’s teeth?
a)
Convex mirror
b)
Plane mirror
c)
Concave mirror
d)
Any spherical mirror
|
|
Sagar chauhan answered |
A small mouth mirror is used by a dentist to examine a small cavity in a patient.
CASE-II
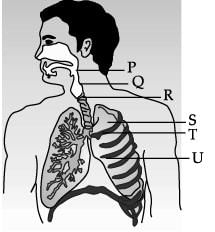
Study the diagram of human respiratory system and answer the following questions.
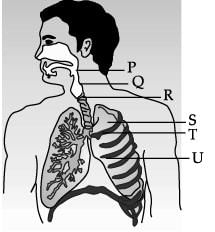
Which of these is the function of balloon like structure present in lungs?- a)Exchange of gases
- b)Absorption of nutrients
- c)Transport of food
- d)Removal of waste materials
Correct answer is option 'A'. Can you explain this answer?
CASE-II
Study the diagram of human respiratory system and answer the following questions.

Which of these is the function of balloon like structure present in lungs?
Study the diagram of human respiratory system and answer the following questions.

Which of these is the function of balloon like structure present in lungs?
a)
Exchange of gases
b)
Absorption of nutrients
c)
Transport of food
d)
Removal of waste materials
|
|
Ananya Das answered |
The balloon like structure called alveoli allow oxygen and carbon dioxide to move between the lungs and the blood-stream.
On the basis of sequence of reactions, identify the most and least reactive elements.
A + BX → AX + B
C + AY → CY + A - a)Most reactive: C; Least reactive: B
- b)Most reactive: B; Least reactive: C
- c)Most reactive: A; Least reactive: B
- d)Most reactive: B; Least reactive: A
Correct answer is option 'A'. Can you explain this answer?
On the basis of sequence of reactions, identify the most and least reactive elements.
A + BX → AX + B
C + AY → CY + A
A + BX → AX + B
C + AY → CY + A
a)
Most reactive: C; Least reactive: B
b)
Most reactive: B; Least reactive: C
c)
Most reactive: A; Least reactive: B
d)
Most reactive: B; Least reactive: A
|
|
Naina Sharma answered |
The most reactive metal is C and the least reactive metal is B.
Study the given experimental set-up carefully. Based on the diagram, Choose the correct observation(s) ?
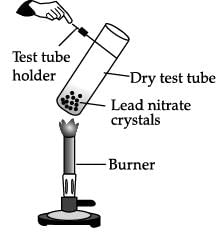
- a)A double decomposition reaction takes place.
- b)Brown fumes of NO2 are evolved.
- c)Red residue is left behind in the test tube.
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
Study the given experimental set-up carefully. Based on the diagram, Choose the correct observation(s) ?


a)
A double decomposition reaction takes place.
b)
Brown fumes of NO2 are evolved.
c)
Red residue is left behind in the test tube.
d)
All of these
|
|
Radha Iyer answered |
On heating, lead nitrate decomposes to give yellow lead monoxide, nitrogen dioxide (emission of brown fumes) and oxygen gas. The reaction that takes place is :


CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
Identify the types of chemical reaction occurring during the combustion of fuel:- a)Oxidation & Endothermic reaction
- b)Decomposition & Exothermic reaction
- c)Oxidation & Exothermic reaction
- d)Combination & Endothermic reaction
Correct answer is option 'C'. Can you explain this answer?
CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
Identify the types of chemical reaction occurring during the combustion of fuel:
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
Identify the types of chemical reaction occurring during the combustion of fuel:
a)
Oxidation & Endothermic reaction
b)
Decomposition & Exothermic reaction
c)
Oxidation & Exothermic reaction
d)
Combination & Endothermic reaction
|
|
Kiran Mehta answered |
The addition of oxygen to a substance or removal of hydrogen from a substance is called oxidation. The reaction in which the heat energy is produced is called exothermic reaction.
If a beam of red light and a beam of violet light are incident at the same angle on the inclined surface of a prism from air medium and produce angles of refraction r and v respectively, which of the following is correct?- a)r = v
- b)r > v
- c)r = 1/v
- d)r < v
Correct answer is option 'D'. Can you explain this answer?
If a beam of red light and a beam of violet light are incident at the same angle on the inclined surface of a prism from air medium and produce angles of refraction r and v respectively, which of the following is correct?
a)
r = v
b)
r > v
c)
r = 1/v
d)
r < v

|
Manshi Yadav answered |
D
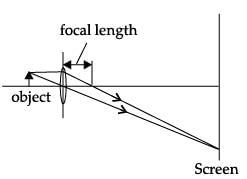
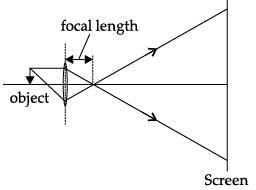
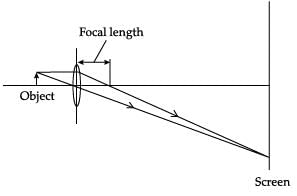
A student obtained a sharp image of a candle flame placed at the distant end of the laboratory table on a screen using a concave mirror to determine its focal length. The teacher suggested him to focus a distant building about 1 km far from the laboratory, for getting more correct value of the focal length. In order to focus the distant building on the same screen the student should slightly move the:- a)mirror away from the screen
- b)screen away from the mirror
- c)screen towards the mirror
- d)screen towards the building
Correct answer is option 'C'. Can you explain this answer?
A student obtained a sharp image of a candle flame placed at the distant end of the laboratory table on a screen using a concave mirror to determine its focal length. The teacher suggested him to focus a distant building about 1 km far from the laboratory, for getting more correct value of the focal length. In order to focus the distant building on the same screen the student should slightly move the:
a)
mirror away from the screen
b)
screen away from the mirror
c)
screen towards the mirror
d)
screen towards the building
|
|
Nilesh Sen answered |
To understand why the student needs to move the screen towards the mirror in order to focus the distant building on the same screen, we need to consider the properties of concave mirrors and how they form images.
1. Concave Mirror:
- A concave mirror is a curved mirror that curves inward.
- It has a reflective surface on the inner side, and the outer side is transparent.
- Concave mirrors are converging mirrors, meaning they bring parallel rays of light together at a specific point called the focus.
2. Focal Length:
- The focal length of a concave mirror is the distance between the mirror and its focus.
- It determines the amount of curvature of the mirror.
3. Image Formation:
- When an object is placed in front of a concave mirror, it forms an image.
- The properties of the image depend on the position of the object relative to the focal point.
4. Candle Flame Image:
- In the given scenario, the student obtained a sharp image of the candle flame placed at the distant end of the laboratory table on a screen.
- This means the screen was placed at the position where the image of the candle flame was formed.
- The distance between the screen and the concave mirror is equal to the focal length of the mirror.
5. Focusing a Distant Building:
- To determine the more correct value of the focal length, the teacher suggested the student focus a distant building about 1 km away from the laboratory.
- When focusing a distant object, the rays of light coming from that object can be considered as parallel rays.
- Parallel rays of light incident on a concave mirror appear to come from the focus after reflection.
6. Moving the Screen:
- When the student focuses the distant building on the same screen, they need to adjust the position of the screen relative to the mirror.
- Since the rays of light from the distant building are parallel, they will converge to a point at the focus of the concave mirror.
- In order to bring this point of convergence onto the screen, the screen needs to be moved towards the mirror.
- This adjustment allows the image of the distant building to be formed on the screen.
Therefore, to focus the distant building on the same screen, the student should slightly move the screen towards the mirror (option C).
1. Concave Mirror:
- A concave mirror is a curved mirror that curves inward.
- It has a reflective surface on the inner side, and the outer side is transparent.
- Concave mirrors are converging mirrors, meaning they bring parallel rays of light together at a specific point called the focus.
2. Focal Length:
- The focal length of a concave mirror is the distance between the mirror and its focus.
- It determines the amount of curvature of the mirror.
3. Image Formation:
- When an object is placed in front of a concave mirror, it forms an image.
- The properties of the image depend on the position of the object relative to the focal point.
4. Candle Flame Image:
- In the given scenario, the student obtained a sharp image of the candle flame placed at the distant end of the laboratory table on a screen.
- This means the screen was placed at the position where the image of the candle flame was formed.
- The distance between the screen and the concave mirror is equal to the focal length of the mirror.
5. Focusing a Distant Building:
- To determine the more correct value of the focal length, the teacher suggested the student focus a distant building about 1 km away from the laboratory.
- When focusing a distant object, the rays of light coming from that object can be considered as parallel rays.
- Parallel rays of light incident on a concave mirror appear to come from the focus after reflection.
6. Moving the Screen:
- When the student focuses the distant building on the same screen, they need to adjust the position of the screen relative to the mirror.
- Since the rays of light from the distant building are parallel, they will converge to a point at the focus of the concave mirror.
- In order to bring this point of convergence onto the screen, the screen needs to be moved towards the mirror.
- This adjustment allows the image of the distant building to be formed on the screen.
Therefore, to focus the distant building on the same screen, the student should slightly move the screen towards the mirror (option C).
Choose the correct statement.- a)Energy produced during respiration is stored in the form of ATP in animals.
- b)ADP has high energy content as compared to ATP.
- c)Respiration is just same as photosynthesis.
- d)All the above
Correct answer is option 'A'. Can you explain this answer?
Choose the correct statement.
a)
Energy produced during respiration is stored in the form of ATP in animals.
b)
ADP has high energy content as compared to ATP.
c)
Respiration is just same as photosynthesis.
d)
All the above
|
|
Anita Menon answered |
ATP is the higher energy form (the recharged battery) while ADP is the lower energy form. Respiration is catabolic while photosynthesis is an anabolic process. In respiration, glucose is broken down into water and carbon dioxide (and energy). In contrast carbon dioxide and water combine in the presence of sunlight to produce glucose and oxygen during photosynthesis.
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?- a)Baking powder
- b)Lime
- c)Ammonium hydroxide solution
- d)Hydrochloric acid
Correct answer is option 'D'. Can you explain this answer?
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
a)
Baking powder
b)
Lime
c)
Ammonium hydroxide solution
d)
Hydrochloric acid
|
|
Aditya Shah answered |
The solution turns red litmus to blue it means that it is basic in nature, to reverse the change acid should be added to it. Thus, out of the given options hydrochloric acid is the correct answer.
Which of the following take place after we exercise? - a)Out body needs more oxygen.
- b)Our body needs to replace the energy used.
- c)Our body needs to get rid of excess carbon dioxide.
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Which of the following take place after we exercise?
a)
Out body needs more oxygen.
b)
Our body needs to replace the energy used.
c)
Our body needs to get rid of excess carbon dioxide.
d)
All of these
|
|
Ramya menon answered |
After we exercise, several physiological changes occur in our body to meet the increased demands of physical activity. These changes include increased oxygen consumption, energy expenditure, and carbon dioxide production. Therefore, all of the given options are correct.
Increased Oxygen Consumption
During exercise, our muscles require more energy to contract and perform work. This increased energy demand is met by an increased oxygen consumption. Oxygen is necessary for the breakdown of glucose and fatty acids in our cells through a process called aerobic respiration. This process produces adenosine triphosphate (ATP), which is the primary energy source for our muscles. Therefore, our body needs more oxygen to fuel this energy production during exercise.
Energy Replacement
As we exercise, our body utilizes stored energy sources such as glycogen (in the muscles and liver) and adipose tissue (body fat). These energy sources are broken down to release ATP and provide energy for muscle contractions. After exercise, our body needs to replenish these energy stores to prepare for future physical activity. This is achieved through the consumption of carbohydrates and fats in our diet. These nutrients are converted into glycogen and stored in the muscles and liver for later use.
Carbon Dioxide Elimination
During exercise, our muscles produce carbon dioxide as a byproduct of ATP production through aerobic respiration. This carbon dioxide needs to be eliminated from our body to maintain proper pH levels and prevent the buildup of waste products. The excess carbon dioxide is transported in our bloodstream to the lungs, where it is exhaled. This process is known as respiration, and it helps to maintain the balance of carbon dioxide and oxygen levels in our body.
In summary, after exercise, our body requires more oxygen to meet the increased energy demands, it needs to replace the energy used by replenishing glycogen stores, and it needs to eliminate excess carbon dioxide produced during exercise. Therefore, all of the given options (a, b, and c) are correct.
Increased Oxygen Consumption
During exercise, our muscles require more energy to contract and perform work. This increased energy demand is met by an increased oxygen consumption. Oxygen is necessary for the breakdown of glucose and fatty acids in our cells through a process called aerobic respiration. This process produces adenosine triphosphate (ATP), which is the primary energy source for our muscles. Therefore, our body needs more oxygen to fuel this energy production during exercise.
Energy Replacement
As we exercise, our body utilizes stored energy sources such as glycogen (in the muscles and liver) and adipose tissue (body fat). These energy sources are broken down to release ATP and provide energy for muscle contractions. After exercise, our body needs to replenish these energy stores to prepare for future physical activity. This is achieved through the consumption of carbohydrates and fats in our diet. These nutrients are converted into glycogen and stored in the muscles and liver for later use.
Carbon Dioxide Elimination
During exercise, our muscles produce carbon dioxide as a byproduct of ATP production through aerobic respiration. This carbon dioxide needs to be eliminated from our body to maintain proper pH levels and prevent the buildup of waste products. The excess carbon dioxide is transported in our bloodstream to the lungs, where it is exhaled. This process is known as respiration, and it helps to maintain the balance of carbon dioxide and oxygen levels in our body.
In summary, after exercise, our body requires more oxygen to meet the increased energy demands, it needs to replace the energy used by replenishing glycogen stores, and it needs to eliminate excess carbon dioxide produced during exercise. Therefore, all of the given options (a, b, and c) are correct.
CASE-II
Study the diagram of human respiratory system and answer the following questions.
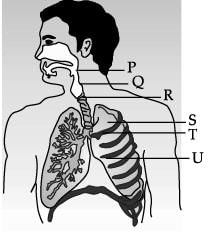
Which of these organ is surrounded by cartilaginous rings? - a)P
- b)Q
- c)R
- d)S
Correct answer is option 'C'. Can you explain this answer?
CASE-II
Study the diagram of human respiratory system and answer the following questions.

Which of these organ is surrounded by cartilaginous rings?
Study the diagram of human respiratory system and answer the following questions.

Which of these organ is surrounded by cartilaginous rings?
a)
P
b)
Q
c)
R
d)
S
|
|
Krishna Iyer answered |
R (Trachea) is supported by rings of cartilage.
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): Sodium carbonate is a basic salt.
Reason (R): It is a salt of weak acid and strong base.- a)Both A and R are true and R is the correct explanation of A.
- b)Both A and R are true but R is NOT the correct explanation of A.
- c)A is true but R is false.
- d)A is false and R is true.
Correct answer is option 'A'. Can you explain this answer?
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): Sodium carbonate is a basic salt.
Reason (R): It is a salt of weak acid and strong base.
Assertion (A): Sodium carbonate is a basic salt.
Reason (R): It is a salt of weak acid and strong base.
a)
Both A and R are true and R is the correct explanation of A.
b)
Both A and R are true but R is NOT the correct explanation of A.
c)
A is true but R is false.
d)
A is false and R is true.
|
|
Anita Menon answered |
Sodium carbonate is a basic salt because it is a salt of weak acid and strong base.
A negative sign in the magnification value indicate that the image is _________- a)Real and inverted
- b)Real and erect
- c)Virtual and erect
- d)Virtual and inverted
Correct answer is option 'A'. Can you explain this answer?
A negative sign in the magnification value indicate that the image is _________
a)
Real and inverted
b)
Real and erect
c)
Virtual and erect
d)
Virtual and inverted
|
|
Aditya Shah answered |
A negative sign in the magnification value indicate that the image is real and inverted.
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): An equation is the shorthand representation of a chemical reaction.
Reason (R): A chemical reaction is a process in which a chemical substance is transformed into another chemical substance.- a)Both A and R are true and R is the correct explanation of A.
- b)Both A and R are true but R is NOT the correct explanation of A.
- c)A is true but R is false.
- d)A is false and R is true.
Correct answer is option 'B'. Can you explain this answer?
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): An equation is the shorthand representation of a chemical reaction.
Reason (R): A chemical reaction is a process in which a chemical substance is transformed into another chemical substance.
Assertion (A): An equation is the shorthand representation of a chemical reaction.
Reason (R): A chemical reaction is a process in which a chemical substance is transformed into another chemical substance.
a)
Both A and R are true and R is the correct explanation of A.
b)
Both A and R are true but R is NOT the correct explanation of A.
c)
A is true but R is false.
d)
A is false and R is true.
|
|
Sanya verma answered |
Assertion (A) and Reason (R) in Chemistry:
Assertion (A): An equation is the shorthand representation of a chemical reaction.
Reason (R): A chemical reaction is a process in which a chemical substance is transformed into another chemical substance.
Explanation:
Chemical Equations:
- In chemistry, a chemical equation is a symbolic representation of a chemical reaction. It uses chemical symbols and formulas to describe the reactants and products involved in the reaction.
- Chemical equations are used to convey information about the substances involved, the ratio in which they react, and the products that are formed.
Chemical Reactions:
- A chemical reaction is a process in which one or more substances (reactants) undergo chemical changes to form new substances (products).
- During a chemical reaction, the atoms of the reactant molecules rearrange to form new chemical bonds, resulting in the formation of different substances.
- The reactants are written on the left side of the chemical equation, and the products are written on the right side.
Explanation of Assertion (A) and Reason (R):
- Assertion (A) states that an equation is the shorthand representation of a chemical reaction. This is true because a chemical equation represents the reactants and products involved in a chemical reaction using symbols and formulas.
- Reason (R) states that a chemical reaction is a process in which a chemical substance is transformed into another chemical substance. This is also true because during a chemical reaction, the reactant molecules undergo rearrangement of atoms to form new products.
Correct Answer:
- The correct answer is option 'B': Both A and R are true but R is NOT the correct explanation of A.
- Although both the assertion and reason are true, the reason does not provide a direct or specific explanation for the assertion.
- The reason states a general fact about chemical reactions, but it does not specifically explain why an equation is considered a shorthand representation of a chemical reaction.
- The assertion is true because a chemical equation does represent a chemical reaction, but the reason does not provide a direct explanation for this fact.
Conclusion:
- In summary, an equation is indeed a shorthand representation of a chemical reaction, and a chemical reaction involves the transformation of chemical substances. However, the reason does not provide a specific explanation for the assertion, making option 'B' the correct answer.
Assertion (A): An equation is the shorthand representation of a chemical reaction.
Reason (R): A chemical reaction is a process in which a chemical substance is transformed into another chemical substance.
Explanation:
Chemical Equations:
- In chemistry, a chemical equation is a symbolic representation of a chemical reaction. It uses chemical symbols and formulas to describe the reactants and products involved in the reaction.
- Chemical equations are used to convey information about the substances involved, the ratio in which they react, and the products that are formed.
Chemical Reactions:
- A chemical reaction is a process in which one or more substances (reactants) undergo chemical changes to form new substances (products).
- During a chemical reaction, the atoms of the reactant molecules rearrange to form new chemical bonds, resulting in the formation of different substances.
- The reactants are written on the left side of the chemical equation, and the products are written on the right side.
Explanation of Assertion (A) and Reason (R):
- Assertion (A) states that an equation is the shorthand representation of a chemical reaction. This is true because a chemical equation represents the reactants and products involved in a chemical reaction using symbols and formulas.
- Reason (R) states that a chemical reaction is a process in which a chemical substance is transformed into another chemical substance. This is also true because during a chemical reaction, the reactant molecules undergo rearrangement of atoms to form new products.
Correct Answer:
- The correct answer is option 'B': Both A and R are true but R is NOT the correct explanation of A.
- Although both the assertion and reason are true, the reason does not provide a direct or specific explanation for the assertion.
- The reason states a general fact about chemical reactions, but it does not specifically explain why an equation is considered a shorthand representation of a chemical reaction.
- The assertion is true because a chemical equation does represent a chemical reaction, but the reason does not provide a direct explanation for this fact.
Conclusion:
- In summary, an equation is indeed a shorthand representation of a chemical reaction, and a chemical reaction involves the transformation of chemical substances. However, the reason does not provide a specific explanation for the assertion, making option 'B' the correct answer.
How will you protect yourself from the heat generated while diluting a concentrated acid?- a)By adding acid to water with constant stirring.
- b)By adding water to acid with constant stirring.
- c)By adding water to acid followed by base.
- d)By adding base to acid with constant stirring.
Correct answer is option 'A'. Can you explain this answer?
How will you protect yourself from the heat generated while diluting a concentrated acid?
a)
By adding acid to water with constant stirring.
b)
By adding water to acid with constant stirring.
c)
By adding water to acid followed by base.
d)
By adding base to acid with constant stirring.
|
|
Sudha gupta answered |
The process of dissolving an acid or a base in water is a highly exothermic one. The acid must always be added slowly to water with constant stirring. It water is added to concentrated acid, the heat generated may cause the mixture to splash out and cause burns.
CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
‘A student while walking on the road observed that a cloud of black smoke belched out from the exhaust stack of moving trucks on the road.’ Choose the correct reason for the production of black smoke:- a)Limited supply of air leads to incomplete combustion of fuel.
- b)Rich supply of air leads to complete combustion of fuel.
- c)Rich supply of air leads to a combination reaction.
- d)Limited supply of air leads to complete combustion of fuel.
Correct answer is option 'A'. Can you explain this answer?
CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
‘A student while walking on the road observed that a cloud of black smoke belched out from the exhaust stack of moving trucks on the road.’ Choose the correct reason for the production of black smoke:
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
‘A student while walking on the road observed that a cloud of black smoke belched out from the exhaust stack of moving trucks on the road.’ Choose the correct reason for the production of black smoke:
a)
Limited supply of air leads to incomplete combustion of fuel.
b)
Rich supply of air leads to complete combustion of fuel.
c)
Rich supply of air leads to a combination reaction.
d)
Limited supply of air leads to complete combustion of fuel.
|
|
Meera Rana answered |
The limited supply of air leads to incomplete combustion of fuel, which in turn leads to the production of black smoke.
In case of concave mirror, the image distance is _________ when image is formed in front of the mirror and _________________ when the image is formed behind the mirror.- a)positive, negative
- b)negative, negative
- c)negative, positive
- d)positive, positive
Correct answer is option 'C'. Can you explain this answer?
In case of concave mirror, the image distance is _________ when image is formed in front of the mirror and _________________ when the image is formed behind the mirror.
a)
positive, negative
b)
negative, negative
c)
negative, positive
d)
positive, positive
|
|
Pooja Shah answered |
If an image formed behind the concave mirror, the object distance is positive but if an image is formed in front of the mirror, the image distance is negative.
In which of the following, the image of an object placed at infinity will be highly diminished and point sized?- a)Concave mirror only
- b)Convex mirror only
- c)Convex lens only
- d)Convex mirror, convex mirror, concave lens and convex lens
Correct answer is option 'D'. Can you explain this answer?
In which of the following, the image of an object placed at infinity will be highly diminished and point sized?
a)
Concave mirror only
b)
Convex mirror only
c)
Convex lens only
d)
Convex mirror, convex mirror, concave lens and convex lens
|
|
Aditya Shah answered |
All the incident rays will be parallel to the principal axis and after reflection or refraction they actually meet or appear to meet at the principal focus.
CASE-II
Study the diagram of human respiratory system and answer the following questions.
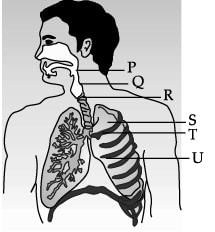
The balloon like structures present in ‘S’ is: - a)Nephron
- b)Alveoli
- c)Bronchi
- d)Bronchiole
Correct answer is option 'B'. Can you explain this answer?
CASE-II
Study the diagram of human respiratory system and answer the following questions.

The balloon like structures present in ‘S’ is:
Study the diagram of human respiratory system and answer the following questions.

The balloon like structures present in ‘S’ is:
a)
Nephron
b)
Alveoli
c)
Bronchi
d)
Bronchiole

|
Siddhi Talreja answered |
Correct option is b because the balloon like structure present in lungs is Alveoli. Exchange of gases takes place in Alveoli.
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): A beam of white light gives a spectrum on passing through a glass prism.
Reason (R): Speed of light outside the prism is different from the speed of light inside the prism.- a)Both A and R are true and R is the correct explanation of A.
- b)Both A and R are true but R is NOT the correct explanation of A.
- c)A is true but R is false.
- d)A is false and R is true.
Correct answer is option 'D'. Can you explain this answer?
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): A beam of white light gives a spectrum on passing through a glass prism.
Reason (R): Speed of light outside the prism is different from the speed of light inside the prism.
Assertion (A): A beam of white light gives a spectrum on passing through a glass prism.
Reason (R): Speed of light outside the prism is different from the speed of light inside the prism.
a)
Both A and R are true and R is the correct explanation of A.
b)
Both A and R are true but R is NOT the correct explanation of A.
c)
A is true but R is false.
d)
A is false and R is true.
|
|
Amit Kumar answered |
A beam of white light gives a spectrum on passing through a glass prism. The band of seven colours formed due to dispersion of white light is called spectrum. Dispersion takes place because the refractive index of medium for different colour is different. Therefore when white light travels from air to air, refractive index remains same and no dispersion occurs.
CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
On the basis of evolution/absorption of energy, which of the following processes are similar to combustion of fuel?
(i) Photosynthesis in plants
(ii) Respiration in the human body
(iii) Decomposition of vegetable matter
(iv) Decomposition of ferrous sulphate.- a)(ii) and (iii)
- b)(i) and (ii)
- c)(iii) and (iv)
- d)(ii) and (i)
Correct answer is option 'A'. Can you explain this answer?
CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
On the basis of evolution/absorption of energy, which of the following processes are similar to combustion of fuel?
(i) Photosynthesis in plants
(ii) Respiration in the human body
(iii) Decomposition of vegetable matter
(iv) Decomposition of ferrous sulphate.
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
On the basis of evolution/absorption of energy, which of the following processes are similar to combustion of fuel?
(i) Photosynthesis in plants
(ii) Respiration in the human body
(iii) Decomposition of vegetable matter
(iv) Decomposition of ferrous sulphate.
a)
(ii) and (iii)
b)
(i) and (ii)
c)
(iii) and (iv)
d)
(ii) and (i)
|
|
Avinash Patel answered |
The process of respiration in the human body and decomposition of vegetable matter involves evolution of energy.
CASE-III
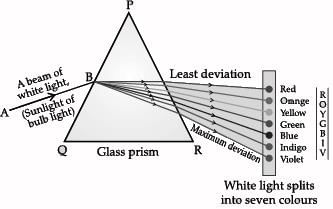
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.
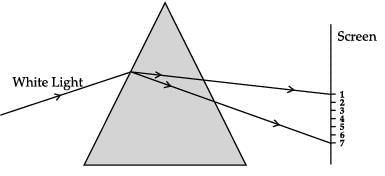
The colour at positions marked 1 and 3 are similar to the colour of ‘turmeric’ and the colour of ‘Chilli powder’, respectively. Choose the correct option.- a)3- turmeric (yellow), 1- chilli powder (red)
- b)1- turmeric (red), 1- chilli powder (yellow)
- c)Both 1 and 3 are red in colour
- d)Both 1 and 3 are yellow in colour.
Correct answer is option 'B'. Can you explain this answer?
CASE-III
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

The colour at positions marked 1 and 3 are similar to the colour of ‘turmeric’ and the colour of ‘Chilli powder’, respectively. Choose the correct option.
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

The colour at positions marked 1 and 3 are similar to the colour of ‘turmeric’ and the colour of ‘Chilli powder’, respectively. Choose the correct option.
a)
3- turmeric (yellow), 1- chilli powder (red)
b)
1- turmeric (red), 1- chilli powder (yellow)
c)
Both 1 and 3 are red in colour
d)
Both 1 and 3 are yellow in colour.
|
|
Meera Rana answered |
The colour at position marked 1 is red while colour at position marked 3 is yellow in colour.

You are given water, mustard oil, glycerine, and kerosene. In which of these media, a ray of light incident obliquely at some angle would bend the most.- a)Kerosene
- b)Water
- c)Mustard oil
- d)Glycerine
Correct answer is option 'D'. Can you explain this answer?
You are given water, mustard oil, glycerine, and kerosene. In which of these media, a ray of light incident obliquely at some angle would bend the most.
a)
Kerosene
b)
Water
c)
Mustard oil
d)
Glycerine
|
|
Ansh dey answered |
Refractive index for water, mustard oil, glycerine and kerosene are:
μw = 1.33
μm = 1.47
μg = 1.473
μk = 1.44
Since refractive index of glycerine is highest, ray bends the most in case of glycerine.
μw = 1.33
μm = 1.47
μg = 1.473
μk = 1.44
Since refractive index of glycerine is highest, ray bends the most in case of glycerine.
Which of the following can make a parallel beam of light when light from a point source is incident on it?- a)Concave mirror as well as convex lens.
- b)Convex mirror as well as concave lens.
- c)Two plane mirrors placed at 90° to each others.
- d)Concave mirror as well as concave lens.
Correct answer is option 'A'. Can you explain this answer?
Which of the following can make a parallel beam of light when light from a point source is incident on it?
a)
Concave mirror as well as convex lens.
b)
Convex mirror as well as concave lens.
c)
Two plane mirrors placed at 90° to each others.
d)
Concave mirror as well as concave lens.
|
|
Ash Mann answered |
Correct option is A) because
When light rays from a point source is incident on concave mirror and convex lens, emergent beams are parallel and the image is formed at infinity.
Convex mirror and concave lens on the other hand always form a virtual image. Emergent beams from these are not parallel but diverging when the object is kept at focus.
In your laboratory you trace the path of light rays through a glass slab for different values of angle of incidence (∠i) and in each case measure the values of the corresponding angle of refraction (∠r) and angle of emergence (∠e).
On the basis of your observation your correct conclusion is:- a)∠i is more than ∠r, but nearly equal to ∠e
- b)∠i is less than ∠r, but nearly equal to ∠e
- c)∠i is more than ∠e, but nearly equal to ∠r
- d)∠i is less than ∠e, but nearly equal to ∠r
Correct answer is option 'C'. Can you explain this answer?
In your laboratory you trace the path of light rays through a glass slab for different values of angle of incidence (∠i) and in each case measure the values of the corresponding angle of refraction (∠r) and angle of emergence (∠e).
On the basis of your observation your correct conclusion is:
On the basis of your observation your correct conclusion is:
a)
∠i is more than ∠r, but nearly equal to ∠e
b)
∠i is less than ∠r, but nearly equal to ∠e
c)
∠i is more than ∠e, but nearly equal to ∠r
d)
∠i is less than ∠e, but nearly equal to ∠r
|
|
Abhi verma answered |
Understanding Light Refraction through a Glass Slab
When light passes through a glass slab, its behavior is governed by the laws of refraction, primarily described by Snell's Law. In your experiment, you observed the relationships between the angle of incidence (i), angle of refraction (r), and angle of emergence (e).
Key Observations
- Angle of Incidence & Angle of Refraction:
- When light enters a denser medium like glass, it slows down, resulting in the angle of refraction being less than the angle of incidence. This means i > r.
- Angle of Emergence:
- As light exits the glass slab back into air, it speeds up again, and according to Snell's Law, the angle of emergence (e) is similar to the angle of incidence due to the symmetry of refraction at the air-glass interface. Hence, e ≈ i.
Conclusion
From these observations, we can derive the following relationships:
- i > r: The angle of incidence is greater than the angle of refraction.
- e ≈ i: The angle of emergence is nearly equal to the angle of incidence, as light exits the slab.
Thus, in your specific case, the correct conclusion is that the angle of incidence is more than the angle of emergence but nearly equal to the angle of refraction, which aligns with option 'C':
Final Answer
- i is more than e, but nearly equal to r: Hence, option 'C' is correct.
This understanding exemplifies the behavior of light as it transitions between different media, emphasizing the principles of refraction in optics.
When light passes through a glass slab, its behavior is governed by the laws of refraction, primarily described by Snell's Law. In your experiment, you observed the relationships between the angle of incidence (i), angle of refraction (r), and angle of emergence (e).
Key Observations
- Angle of Incidence & Angle of Refraction:
- When light enters a denser medium like glass, it slows down, resulting in the angle of refraction being less than the angle of incidence. This means i > r.
- Angle of Emergence:
- As light exits the glass slab back into air, it speeds up again, and according to Snell's Law, the angle of emergence (e) is similar to the angle of incidence due to the symmetry of refraction at the air-glass interface. Hence, e ≈ i.
Conclusion
From these observations, we can derive the following relationships:
- i > r: The angle of incidence is greater than the angle of refraction.
- e ≈ i: The angle of emergence is nearly equal to the angle of incidence, as light exits the slab.
Thus, in your specific case, the correct conclusion is that the angle of incidence is more than the angle of emergence but nearly equal to the angle of refraction, which aligns with option 'C':
Final Answer
- i is more than e, but nearly equal to r: Hence, option 'C' is correct.
This understanding exemplifies the behavior of light as it transitions between different media, emphasizing the principles of refraction in optics.
The chemical reaction between copper and oxygen can be categorized as:- a)Displacement reaction
- b)Decomposition reaction
- c)Combination reaction
- d)Double displacement reaction
Correct answer is option 'C'. Can you explain this answer?
The chemical reaction between copper and oxygen can be categorized as:
a)
Displacement reaction
b)
Decomposition reaction
c)
Combination reaction
d)
Double displacement reaction
|
|
Beauty Dubey answered |
Copper+oxygen=copper oxide.
And it is categorised in the category of combination reaction.
So, answer (c)is correct.
And it is categorised in the category of combination reaction.
So, answer (c)is correct.
Why is it important to balance a skeletal chemical equation?- a)To verify law of conservation of energy.
- b)To verify the law of constant proportion.
- c)To verify the law of conservation of mass.
- d)To verify the law of conservation of momentum.
Correct answer is option 'C'. Can you explain this answer?
Why is it important to balance a skeletal chemical equation?
a)
To verify law of conservation of energy.
b)
To verify the law of constant proportion.
c)
To verify the law of conservation of mass.
d)
To verify the law of conservation of momentum.

|
Soumyaneel Mukherjee answered |
In a chemical reaction mass is neither created nor destroyed. The total mass of the reactants is always equal to the total mass of the products formed in the reaction. A skeletal equation would defy the law of conservation of mass.There is a change only in the energy of the reactants and products.
CASE-II
Study the diagram of human respiratory system and answer the following questions.
Which of these statements is incorrect regarding human lungs? - a)It is the secondary organ for respiration.
- b)It is located on the two sides of heart.
- c)The membrane that encloses lungs is pleural membrane.
- d)The alveolar epithelium of lungs is non-ciliated epithelium.
Correct answer is option 'A'. Can you explain this answer?
CASE-II
Study the diagram of human respiratory system and answer the following questions.

Which of these statements is incorrect regarding human lungs?
Study the diagram of human respiratory system and answer the following questions.

Which of these statements is incorrect regarding human lungs?
a)
It is the secondary organ for respiration.
b)
It is located on the two sides of heart.
c)
The membrane that encloses lungs is pleural membrane.
d)
The alveolar epithelium of lungs is non-ciliated epithelium.
|
|
Krishna Iyer answered |
Lungs are the primary breathing organ. It is the main respiratory surface available for the exchange of gases (O2 & CO2).
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): The by product of autotrophic nutrition is oxygen.
Reason (R): Oxygen is released into the atmosphere through stomata.- a)Both A and R are true and R is the correct explanation of A.
- b)Both A and R are true but R is NOT the correct explanation of A.
- c)A is true but R is false.
- d)A is false and R is true.
Correct answer is option 'B'. Can you explain this answer?
Directions: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): The by product of autotrophic nutrition is oxygen.
Reason (R): Oxygen is released into the atmosphere through stomata.
Assertion (A): The by product of autotrophic nutrition is oxygen.
Reason (R): Oxygen is released into the atmosphere through stomata.
a)
Both A and R are true and R is the correct explanation of A.
b)
Both A and R are true but R is NOT the correct explanation of A.
c)
A is true but R is false.
d)
A is false and R is true.
|
|
Radha Iyer answered |
Plants require sunlight, water, chlorophyll and carbon dioxide as raw materials for photosynthesis. Oxygen is released as by-product through stomata on leaf.
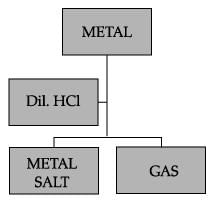
The gas produced when dil. HCl is added to a reactive metal: - a)Oxygen
- b)Nitrogen
- c)Hydrogen
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
The gas produced when dil. HCl is added to a reactive metal:
a)
Oxygen
b)
Nitrogen
c)
Hydrogen
d)
None of the above
|
|
Avinash Patel answered |
Hydrogen gas is produced when dilute HCl is added to a reactive metal.
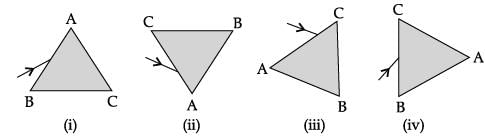
A prism ABC (with BC as base) is placed in different orientations. A narrow beam of white light is incident on the prism as shown in below Figure. In which of the following diagrams, after dispersion, the third colour from the top of the spectrum corresponds to the colour of the sky?
- a)(i)
- b)(ii)
- c)(iii)
- d)(iv)
Correct answer is option 'B'. Can you explain this answer?
A prism ABC (with BC as base) is placed in different orientations. A narrow beam of white light is incident on the prism as shown in below Figure. In which of the following diagrams, after dispersion, the third colour from the top of the spectrum corresponds to the colour of the sky?


a)
(i)
b)
(ii)
c)
(iii)
d)
(iv)
|
|
Bishwanath Patra answered |
We know that after dispersion all lights bend towards base so A.T.Q we observe sky colour at third position from top.in option B. so option B is correct.
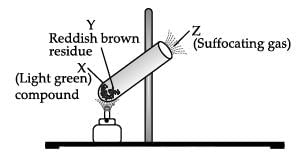
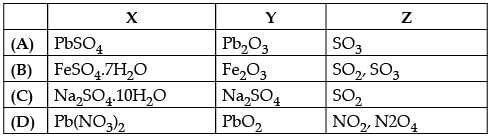
Shyam heated a small amount of light green colored compound X in a test tube. He found that the compound losed some water and then gas Z with suffocating smell comes out. The vapors of gas are collected and dissolved in water. The solution turns blue litmus red. The residue Y left in the test tube turns reddish brown.
Identify X,Y and Z
- a)A
- b)B
- c)C
- d)D
Correct answer is option 'B'. Can you explain this answer?
Shyam heated a small amount of light green colored compound X in a test tube. He found that the compound losed some water and then gas Z with suffocating smell comes out. The vapors of gas are collected and dissolved in water. The solution turns blue litmus red. The residue Y left in the test tube turns reddish brown.

Identify X,Y and Z


Identify X,Y and Z

a)
A
b)
B
c)
C
d)
D
|
|
Radha Iyer answered |
The green colour compound X is FeSO4.7H2O.
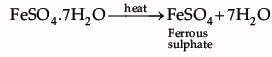
Ferrous sulphate decomposes with the evolution of a gas having a typical odour of burning sulphur. It is a thermal decomposition reaction.
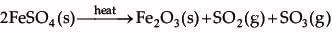
SO2 and SO3 are acidic in nature, turn blue litmus red as they react with water to form acids
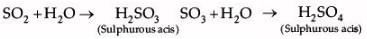
Hence, X is FeSO4.7H2O , Y is Fe2O3 and Z is SO2, SO4

Ferrous sulphate decomposes with the evolution of a gas having a typical odour of burning sulphur. It is a thermal decomposition reaction.
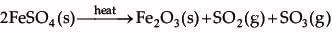
SO2 and SO3 are acidic in nature, turn blue litmus red as they react with water to form acids
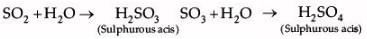
Hence, X is FeSO4.7H2O , Y is Fe2O3 and Z is SO2, SO4
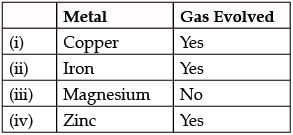
Identify gas A in the following experiment.
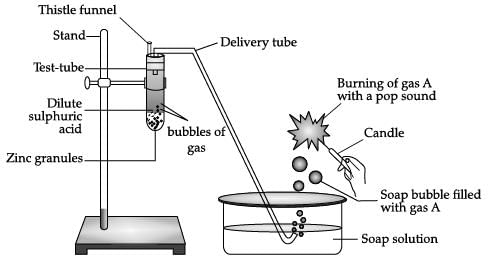
- a)Nitrogen
- b)Hydrogen
- c)Oxygen
- d)Carbon dioxide
Correct answer is option 'B'. Can you explain this answer?
Identify gas A in the following experiment.


a)
Nitrogen
b)
Hydrogen
c)
Oxygen
d)
Carbon dioxide
|
|
Parth Mehta answered |
Copper will not react with dil. HCl.
Mg is highly reactive with dil. HCl.
Mg + 2Hcl ⟶ H2 + Mgcl2.
A cracking sound is heard as hydrogen burns.
When dil. HCl is added to Fe, then ferrous chloride (FeCl2) and hydrogen gas (H2) will be formed.
2HCl + Fe ⟶ FeCl2 + H2.
Mg is highly reactive with dil. HCl.
Mg + 2Hcl ⟶ H2 + Mgcl2.
A cracking sound is heard as hydrogen burns.
When dil. HCl is added to Fe, then ferrous chloride (FeCl2) and hydrogen gas (H2) will be formed.
2HCl + Fe ⟶ FeCl2 + H2.
Which of the following chemical reactions is incorrect?- a)
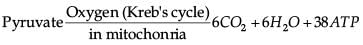
- b)
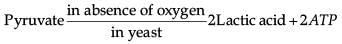
- c)
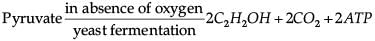
- d)
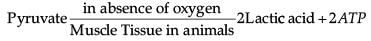
Correct answer is option 'B'. Can you explain this answer?
Which of the following chemical reactions is incorrect?
a)
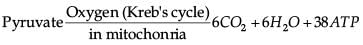
b)
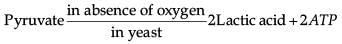
c)
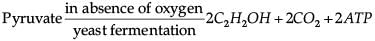
d)
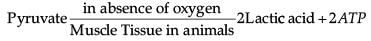
|
|
Amit Sharma answered |
Pyruvate is converted into lactic acid when there is a lack of oxygen in our muscle cells.
CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
Which of the following are the products obtained from the reaction mentioned in the above case?
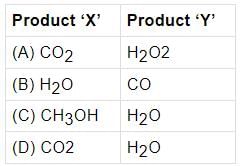
- a)A
- b)B
- c)C
- d)D
Correct answer is option 'D'. Can you explain this answer?
CASE-I
Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
Which of the following are the products obtained from the reaction mentioned in the above case?

Chemistry in Automobiles:
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical "spark",which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) → 16 'X' + Y
Which of the following are the products obtained from the reaction mentioned in the above case?

a)
A
b)
B
c)
C
d)
D
|
|
Amit Kumar answered |
The complete combustion of gasoline in full supply of air results in production of carbon dioxide and water. The chemical reaction is as follows:
2C8H18 (l) + 25O2(g) → 16CO2(g) + 18H2O(g)
2C8H18 (l) + 25O2(g) → 16CO2(g) + 18H2O(g)
Identify the phase of circulation which is represented in the diagram of heart given below. Arrows indicate contraction of the chambers shown.
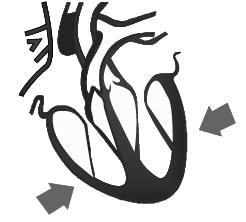
- a)Blood transferred to the right ventricle and left ventricle simultaneously.
- b)Blood is transferred to lungs for oxygenation and is pumped into various organs simultaneously.
- c)Blood transferred to the right auricle and left auricle simultaneously.
- d)Blood is received from lungs after oxygenation and is received from various organs of the body.
Correct answer is option 'B'. Can you explain this answer?
Identify the phase of circulation which is represented in the diagram of heart given below. Arrows indicate contraction of the chambers shown.


a)
Blood transferred to the right ventricle and left ventricle simultaneously.
b)
Blood is transferred to lungs for oxygenation and is pumped into various organs simultaneously.
c)
Blood transferred to the right auricle and left auricle simultaneously.
d)
Blood is received from lungs after oxygenation and is received from various organs of the body.
|
|
Manav kumar answered |
- When it pumps the blood to the organs, the heart is contracted.
- When it receives the blood from the lungs, the heart is relaxed.
- In this figure, contraction in both ventricles is due to the fact that blood is pumping out.
- In the left ventricle, the oxygenated blood is pumping to other parts of the body.
- In the right ventricle, the deoxygenated blood is pumped to lungs for purification of the blood.
Thus, in our case: Blood is transferred to lungs for oxygenation and is pumped into various organs simultaneously.
- When it receives the blood from the lungs, the heart is relaxed.
- In this figure, contraction in both ventricles is due to the fact that blood is pumping out.
- In the left ventricle, the oxygenated blood is pumping to other parts of the body.
- In the right ventricle, the deoxygenated blood is pumped to lungs for purification of the blood.
Thus, in our case: Blood is transferred to lungs for oxygenation and is pumped into various organs simultaneously.
Choose the correct option .
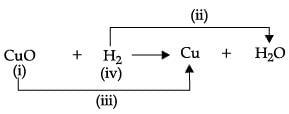
- a)(ii)- oxidation, (iii) – reduction
- b)(ii)- reduction, (iii)- oxidation
- c)(i) – reducing agent, (iv)- oxidizing agent
- d)(i) – undergoes reduction (iv) – undergoes reduction
Correct answer is option 'A'. Can you explain this answer?
Choose the correct option .


a)
(ii)- oxidation, (iii) – reduction
b)
(ii)- reduction, (iii)- oxidation
c)
(i) – reducing agent, (iv)- oxidizing agent
d)
(i) – undergoes reduction (iv) – undergoes reduction
|
|
Amit Sharma answered |
In the given equation, label (i) represent oxidizing agent, (ii) is oxidation, (iii) is reduction and (iv) represents reducing agent.

Opening and closing of stomatal pore depends on:- a)Atmospheric temperature
- b)oxygen concentration around stomata
- c)carbon dioxide concentration around stomata
- d)water content in the guard cells
Correct answer is option 'D'. Can you explain this answer?
Opening and closing of stomatal pore depends on:
a)
Atmospheric temperature
b)
oxygen concentration around stomata
c)
carbon dioxide concentration around stomata
d)
water content in the guard cells

|
Dhruv Chopra answered |
Hii.. As the Guard cells are like Watchman.. If there is too much water content they will swell out and the pore wd be open.. If water is less it would shrink.. Here They are asking the transpiration part not gaseous exchange thing...
Which of the given options correctly represents the Parent acid and base of Calcium Carbonate?
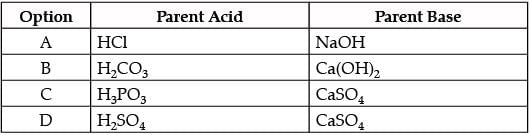
- a)A
- b)B
- c)C
- d)D
Correct answer is option 'B'. Can you explain this answer?
Which of the given options correctly represents the Parent acid and base of Calcium Carbonate?


a)
A
b)
B
c)
C
d)
D
|
|
Rohan Dasgupta answered |
H2CO3 + Ca (OH)2 ⟶ ⟶ CaCO3 + 2H2O
So, Parent acid and parent base for the formation of calcium carbonate is H2CO3 and Ca (OH)2 respectively.
So, Parent acid and parent base for the formation of calcium carbonate is H2CO3 and Ca (OH)2 respectively.
CASE-III
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.
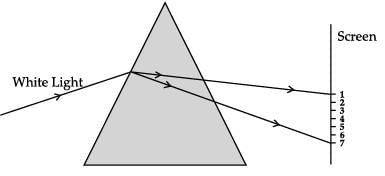
Which two positions correspond to the colour of solution of copper sulphate and signal used to move the vehicles?- a)Position 5 and 4 respectively
- b)Position 1 and 7 respectively
- c)Position 4 and 5 respectively
- d)Position 7 and 1 respectively
Correct answer is option 'A'. Can you explain this answer?
CASE-III
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

Which two positions correspond to the colour of solution of copper sulphate and signal used to move the vehicles?
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

Which two positions correspond to the colour of solution of copper sulphate and signal used to move the vehicles?
a)
Position 5 and 4 respectively
b)
Position 1 and 7 respectively
c)
Position 4 and 5 respectively
d)
Position 7 and 1 respectively
|
|
Meera Rana answered |
Copper sulphate is blue in colour, which is at position 5 where the signal used to move the vehicle is green. Green is at position 5.
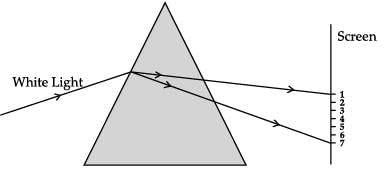
CASE-III
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.
Which of these marked colour deviates the least after refraction through a prism ?- a)7
- b)1
- c)3
- d)5
Correct answer is option 'B'. Can you explain this answer?
CASE-III
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

Which of these marked colour deviates the least after refraction through a prism ?
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

Which of these marked colour deviates the least after refraction through a prism ?
a)
7
b)
1
c)
3
d)
5
|
|
Meera Rana answered |
When a beam of white light gets refracted through a glass prism, it refracts through different angles causing a splitting of white light into its seven constituent colours (VIBGYOR). This gives rise to the formation of the colour spectrum. Violet colour (marked as 7) deviate the most and red colour (marked as 1) deviates the least after refraction through a prism.
The gas X in the given graph is _______.
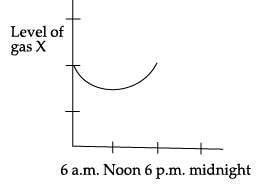
- a)Oxygen
- b)Carbon dioxide
- c)Nitrogen
- d)Water vapour
Correct answer is option 'B'. Can you explain this answer?
The gas X in the given graph is _______.


a)
Oxygen
b)
Carbon dioxide
c)
Nitrogen
d)
Water vapour
|
|
Radha Iyer answered |
Green plants prepare food by taking CO2 from the air by the process of photosynthesis. Photosynthesis occurs more at noon. Hence the carbon dioxide gas is less during noon.
The given experimental set up is used to test a few solutions which contain hydrogen but are not categorized as acids.
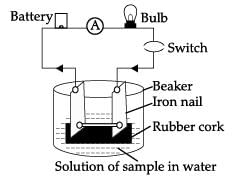
Which of these observation is incorrect?- a)When a solution of glucose is put the bulb does not glow.
- b)When a solution of ethanol is put the bulb does not glow
- c)When a solution of hydrochloric acid is put the bulb glows.
- d)When a solution of sodium hydroxide is put the bulb does not glow.
Correct answer is option 'D'. Can you explain this answer?
The given experimental set up is used to test a few solutions which contain hydrogen but are not categorized as acids.

Which of these observation is incorrect?

Which of these observation is incorrect?
a)
When a solution of glucose is put the bulb does not glow.
b)
When a solution of ethanol is put the bulb does not glow
c)
When a solution of hydrochloric acid is put the bulb glows.
d)
When a solution of sodium hydroxide is put the bulb does not glow.
|
|
Amit Sharma answered |
Glucose and ethanol do not conduct electricity while NaOH and HCl conduct electricity as they produce ions in the solution.
Study the given experimental set up.
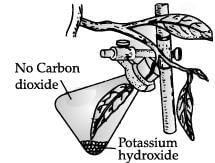
The aim of the given experiment is to prove that _________ is essential for the process of photosynthesis. - a)Sunlight
- b)Carbon dioxide
- c)Water
- d)Chlorophyll
Correct answer is option 'B'. Can you explain this answer?
Study the given experimental set up.

The aim of the given experiment is to prove that _________ is essential for the process of photosynthesis.

The aim of the given experiment is to prove that _________ is essential for the process of photosynthesis.
a)
Sunlight
b)
Carbon dioxide
c)
Water
d)
Chlorophyll
|
|
Amit Kumar answered |
The experimental set up is used to prove that carbon dioxide is essential for photosynthesis.
The graph given below depicts a neutralization reaction (acid + alkali → salt + water).
The pH of a solution changes as we add excess of acid to an alkali.
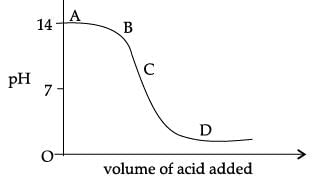
Which letter denotes the area of the graph where both acid and salt are present?- a)A
- b)B
- c)C
- d)D
Correct answer is option 'D'. Can you explain this answer?
The graph given below depicts a neutralization reaction (acid + alkali → salt + water).
The pH of a solution changes as we add excess of acid to an alkali.

Which letter denotes the area of the graph where both acid and salt are present?
The pH of a solution changes as we add excess of acid to an alkali.

Which letter denotes the area of the graph where both acid and salt are present?
a)
A
b)
B
c)
C
d)
D

|
Oshan Aggarwal answered |
C is correct answer bcuz it is a neutralization rxn
Carefully study the diagram of the human respiratory system with labels A, B, C and D. Select the option which gives correct identification and main function and /or characteristic.
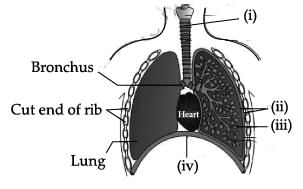
- a)(i) Trachea: It is supported by bony rings for conducting inspired air.
- b)(ii) Ribs: When we breathe out, ribs are lifted.
- c)(iii) Alveoli: Thin-walled sac like structures for exchange of gases.
- d)(iv) Diaphragm: It is pulled up when we breathe in.
Correct answer is option 'C'. Can you explain this answer?
Carefully study the diagram of the human respiratory system with labels A, B, C and D. Select the option which gives correct identification and main function and /or characteristic.


a)
(i) Trachea: It is supported by bony rings for conducting inspired air.
b)
(ii) Ribs: When we breathe out, ribs are lifted.
c)
(iii) Alveoli: Thin-walled sac like structures for exchange of gases.
d)
(iv) Diaphragm: It is pulled up when we breathe in.
|
|
jojin Pandey answered |
Option C is a correct explanation given
The figure given below shows a schematic plan of blood circulation in humans with labels (i) to (iv). Identify the correct label with its functions?
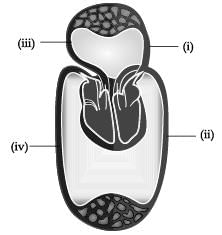
- a)(i) Pulmonary vein - takes impure blood from body part.
- b)(ii) Pulmonary artery - takes blood from lung to heart.
- c)(iii) Aorta - takes blood from heart to body parts.
- d)(iv) Vena cava takes - blood from body parts to right auricle.
Correct answer is option 'D'. Can you explain this answer?
The figure given below shows a schematic plan of blood circulation in humans with labels (i) to (iv). Identify the correct label with its functions?


a)
(i) Pulmonary vein - takes impure blood from body part.
b)
(ii) Pulmonary artery - takes blood from lung to heart.
c)
(iii) Aorta - takes blood from heart to body parts.
d)
(iv) Vena cava takes - blood from body parts to right auricle.
|
|
Samta khanna answered |
Human blood circulation is responsible for the transport of nutrients and oxygen to the different parts of the body. Oxygen-rich blood travels through the pulmonary vein and the left atrium into the left ventricle. So, the partial pressure of oxygen will be very high. The pulmonary artery carries the deoxygenated blood from the right ventricle of the heart to the lungs. Since, the blood is deoxygenated, the blood will have more partial pressure of carbon dioxide than oxygen. Aorta carries the oxygen-rich blood from the left side of the heart to the body. The vena cava is responsible for carrying the deoxygenated blood from the lower and middle parts of the body to the right atrium. The partial pressure of carbon dioxide in vena cava or veins is equal to 45 mm Hg. The partial pressure of oxygen in arterial blood is equal to 100 mm Hg.
CASE-III
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.
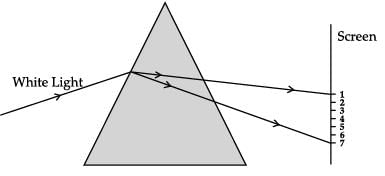
Read the given statements.
I. Light of colour of chilli powder bends the most.
II. The light of colour of brinjal bends the least.
Which of these statement(s) is incorrect?- a)I only
- b)II only
- c)Both I and II
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
CASE-III
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

Read the given statements.
I. Light of colour of chilli powder bends the most.
II. The light of colour of brinjal bends the least.
Which of these statement(s) is incorrect?
A beam of white light falling on a glass prism gets split into seven colours marked 1 to 7. Study the diagram and answer the following questions.

Read the given statements.
I. Light of colour of chilli powder bends the most.
II. The light of colour of brinjal bends the least.
Which of these statement(s) is incorrect?
a)
I only
b)
II only
c)
Both I and II
d)
None of these
|
|
Ishan Choudhury answered |
Both the statements are incorrect. Violet bends the most.
Chapter doubts & questions for CBSE Sample Question Papers for 2021-22 - CBSE Sample Papers For Class 10 2025 is part of Class 10 exam preparation. The chapters have been prepared according to the Class 10 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 10 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of CBSE Sample Question Papers for 2021-22 - CBSE Sample Papers For Class 10 in English & Hindi are available as part of Class 10 exam.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup