All Exams >
Class 5 >
Science Olympiad for Class 5 >
All Questions
All questions of Matter and Materials for Class 5 Exam
The ability to change or to move matter is referred to as:- a)Kinetic theory
- b)Energy
- c)Evaporation
- d)Heating
Correct answer is option 'B'. Can you explain this answer?
The ability to change or to move matter is referred to as:
a)
Kinetic theory
b)
Energy
c)
Evaporation
d)
Heating
|
|
Ajit rana answered |
**Explanation:**
**Energy** refers to the ability to do work or cause change. When it comes to matter, energy can either be transferred or transformed. The ability to change or move matter is a result of energy being transferred or transformed.
**Kinetic Theory:**
The **kinetic theory** explains how particles in matter are in constant motion. It states that all matter is made up of tiny particles (atoms or molecules) that are constantly moving. However, the kinetic theory does not specifically refer to the ability to change or move matter.
**Evaporation:**
**Evaporation** is the process by which a liquid changes into a gas. It occurs when the particles in a liquid gain enough energy to overcome the forces holding them together and escape into the air as vapor. While evaporation involves the transformation of matter, it does not encompass the ability to change or move matter.
**Heating:**
**Heating** involves the transfer of energy from a hotter object to a cooler object. When an object is heated, its particles gain energy and move faster, resulting in an increase in temperature. Heating can cause changes in matter, such as melting or boiling, but it does not directly involve the ability to change or move matter.
**Energy:**
**Energy** is the correct answer because it encompasses the ability to change or move matter. Energy can be transferred or transformed in various forms, such as thermal energy (heat), mechanical energy (motion), chemical energy, and electrical energy. When energy is transferred or transformed, it can cause changes in matter, such as changes in temperature, state (solid, liquid, gas), or position. Therefore, the ability to change or move matter is referred to as energy.
In summary, while the other options mentioned (kinetic theory, evaporation, and heating) are related to energy and matter, they do not specifically encompass the ability to change or move matter. Only energy, in its various forms, has the capacity to cause such changes in matter.
**Energy** refers to the ability to do work or cause change. When it comes to matter, energy can either be transferred or transformed. The ability to change or move matter is a result of energy being transferred or transformed.
**Kinetic Theory:**
The **kinetic theory** explains how particles in matter are in constant motion. It states that all matter is made up of tiny particles (atoms or molecules) that are constantly moving. However, the kinetic theory does not specifically refer to the ability to change or move matter.
**Evaporation:**
**Evaporation** is the process by which a liquid changes into a gas. It occurs when the particles in a liquid gain enough energy to overcome the forces holding them together and escape into the air as vapor. While evaporation involves the transformation of matter, it does not encompass the ability to change or move matter.
**Heating:**
**Heating** involves the transfer of energy from a hotter object to a cooler object. When an object is heated, its particles gain energy and move faster, resulting in an increase in temperature. Heating can cause changes in matter, such as melting or boiling, but it does not directly involve the ability to change or move matter.
**Energy:**
**Energy** is the correct answer because it encompasses the ability to change or move matter. Energy can be transferred or transformed in various forms, such as thermal energy (heat), mechanical energy (motion), chemical energy, and electrical energy. When energy is transferred or transformed, it can cause changes in matter, such as changes in temperature, state (solid, liquid, gas), or position. Therefore, the ability to change or move matter is referred to as energy.
In summary, while the other options mentioned (kinetic theory, evaporation, and heating) are related to energy and matter, they do not specifically encompass the ability to change or move matter. Only energy, in its various forms, has the capacity to cause such changes in matter.
Intermolecular space is least between:
- a)Solid molecules
- b)Gas molecules
- c)Liquid molecules
- d)Both A and B
Correct answer is option 'A'. Can you explain this answer?
Intermolecular space is least between:
a)
Solid molecules
b)
Gas molecules
c)
Liquid molecules
d)
Both A and B
|
|
Sreemoyee Das answered |
Intermolecular space is the space between two molecule or atom. In solids it is very little, in liquids is more the solids but less than liquids and in gases its the maximum.
A substance changes from a solid to a liquid. Which point it has reached?- a)Boiling
- b)Melting
- c)Freezing
- d)Sublimation
Correct answer is option 'B'. Can you explain this answer?
A substance changes from a solid to a liquid. Which point it has reached?
a)
Boiling
b)
Melting
c)
Freezing
d)
Sublimation

|
Prajakta Ghongade answered |
This tempreture change form solid to liquid is called the melting point . Each solid has a set melting point at normal air preessure, but at higher altitudes the melting point lowers .
Two substances, P and Q, were used to fill a syringe. The plunger was then pushed in as shown in the diagram. Which of the following would most likely be P and which would be Q?
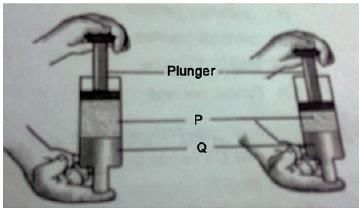

- a)A
- b)B
- c)C
- d)D
Correct answer is option 'C'. Can you explain this answer?
Two substances, P and Q, were used to fill a syringe. The plunger was then pushed in as shown in the diagram. Which of the following would most likely be P and which would be Q?



a)
A
b)
B
c)
C
d)
D
|
|
Stoneridge Institute answered |
Statues are solid. They are made up of solid materials. They cannot be made up of water because water has the tendency to take the shape of its container. It has no shape.
Molecules are the components of:
- a)Solids
- b)All of the these
- c)Solutions
- d)Matter
Correct answer is option 'B'. Can you explain this answer?
Molecules are the components of:
a)
Solids
b)
All of the these
c)
Solutions
d)
Matter
|
|
Pallavi Gupta answered |
**Matter and Its Components**
Matter is everything that occupies space and has mass. It includes everything we can see, touch, or feel, such as solids, liquids, and gases. Matter is made up of tiny particles called atoms and molecules. Atoms are the smallest unit of an element, while molecules are made up of two or more atoms bonded together.
**The Components of Matter**
Matter can be divided into two main components: atoms and molecules. Atoms are the building blocks of matter and cannot be broken down into smaller particles by ordinary chemical means. On the other hand, molecules are composed of two or more atoms that are chemically bonded together.
**Solids and Molecules**
Solids are one of the three main states of matter. They have a definite shape and volume. In solids, the molecules are tightly packed together and held in fixed positions by strong intermolecular forces. These forces keep the molecules close together, allowing solids to maintain their shape.
In a solid, the molecules vibrate around their fixed positions, but they do not move freely like in liquids or gases. The arrangement and motion of molecules in a solid determine its properties, such as density, hardness, and conductivity.
**Solutions and Molecules**
Solutions are homogeneous mixtures of two or more substances. They consist of a solvent (the substance that dissolves other substances) and solutes (the substances that are dissolved in the solvent). The solvent and solutes can be in different states of matter, such as solid solutes dissolved in a liquid solvent.
In a solution, the molecules of the solute are dispersed and evenly distributed throughout the solvent. The solute particles are surrounded by solvent molecules, forming a stable mixture. The interaction between the solvent and solute molecules determines the properties of the solution, such as its concentration and solubility.
**Molecules and Matter**
Since molecules are the building blocks of matter, they are present in all states of matter, including solids, liquids, and gases. Molecules determine the properties and behavior of matter. They are responsible for the physical and chemical properties of substances.
Therefore, molecules are the components of matter. They are the tiny particles that make up everything around us, from the solid objects we interact with to the solutions we use in our daily lives. Hence, the correct answer to the given question is option 'B': Matter.
Matter is everything that occupies space and has mass. It includes everything we can see, touch, or feel, such as solids, liquids, and gases. Matter is made up of tiny particles called atoms and molecules. Atoms are the smallest unit of an element, while molecules are made up of two or more atoms bonded together.
**The Components of Matter**
Matter can be divided into two main components: atoms and molecules. Atoms are the building blocks of matter and cannot be broken down into smaller particles by ordinary chemical means. On the other hand, molecules are composed of two or more atoms that are chemically bonded together.
**Solids and Molecules**
Solids are one of the three main states of matter. They have a definite shape and volume. In solids, the molecules are tightly packed together and held in fixed positions by strong intermolecular forces. These forces keep the molecules close together, allowing solids to maintain their shape.
In a solid, the molecules vibrate around their fixed positions, but they do not move freely like in liquids or gases. The arrangement and motion of molecules in a solid determine its properties, such as density, hardness, and conductivity.
**Solutions and Molecules**
Solutions are homogeneous mixtures of two or more substances. They consist of a solvent (the substance that dissolves other substances) and solutes (the substances that are dissolved in the solvent). The solvent and solutes can be in different states of matter, such as solid solutes dissolved in a liquid solvent.
In a solution, the molecules of the solute are dispersed and evenly distributed throughout the solvent. The solute particles are surrounded by solvent molecules, forming a stable mixture. The interaction between the solvent and solute molecules determines the properties of the solution, such as its concentration and solubility.
**Molecules and Matter**
Since molecules are the building blocks of matter, they are present in all states of matter, including solids, liquids, and gases. Molecules determine the properties and behavior of matter. They are responsible for the physical and chemical properties of substances.
Therefore, molecules are the components of matter. They are the tiny particles that make up everything around us, from the solid objects we interact with to the solutions we use in our daily lives. Hence, the correct answer to the given question is option 'B': Matter.
The most common state of matter in the universe is called:- a)Solid
- b)Liquid
- c)Gas
- d)Plasma
Correct answer is option 'D'. Can you explain this answer?
The most common state of matter in the universe is called:
a)
Solid
b)
Liquid
c)
Gas
d)
Plasma
|
|
Ayush Thakur answered |
The most common state of matter in the universe plasma
so, that the correct answer will be option D) plasma
Matter is made up of these particles:- a)Bubbles
- b)Molecules
- c)Atoms
- d)Circles
Correct answer is option 'C'. Can you explain this answer?
Matter is made up of these particles:
a)
Bubbles
b)
Molecules
c)
Atoms
d)
Circles

|
Learning Enablers answered |
Answer:
Particles that matter is made up of:
- Bubbles: Bubbles are not considered particles of matter. They are formed when a gas is trapped in a liquid or a solid.
- Molecules: Molecules are the smallest units of a compound that retain the chemical properties of that compound. They are made up of two or more atoms bonded together.
- Atoms: Atoms are the basic building blocks of matter. They are the smallest particle of an element that retains the chemical properties of that element. Atoms combine to form molecules.
- Circles: Circles are not particles of matter. They are geometric shapes.
Explanation:
- Matter is anything that has mass and occupies space. It is made up of particles that are constantly in motion.
- The particles that matter is made up of include atoms, molecules, and in some cases, ions.
- Atoms are the fundamental particles of matter. They consist of a nucleus, which contains protons and neutrons, and electrons that orbit around the nucleus.
- Atoms can combine with other atoms to form molecules. Molecules are made up of two or more atoms held together by chemical bonds.
- Bubbles and circles are not particles of matter. Bubbles are formed by the arrangement of molecules in a liquid or solid, and circles are geometric shapes with no physical substance.
- Therefore, the correct answer is C: Atoms.
Particles that matter is made up of:
- Bubbles: Bubbles are not considered particles of matter. They are formed when a gas is trapped in a liquid or a solid.
- Molecules: Molecules are the smallest units of a compound that retain the chemical properties of that compound. They are made up of two or more atoms bonded together.
- Atoms: Atoms are the basic building blocks of matter. They are the smallest particle of an element that retains the chemical properties of that element. Atoms combine to form molecules.
- Circles: Circles are not particles of matter. They are geometric shapes.
Explanation:
- Matter is anything that has mass and occupies space. It is made up of particles that are constantly in motion.
- The particles that matter is made up of include atoms, molecules, and in some cases, ions.
- Atoms are the fundamental particles of matter. They consist of a nucleus, which contains protons and neutrons, and electrons that orbit around the nucleus.
- Atoms can combine with other atoms to form molecules. Molecules are made up of two or more atoms held together by chemical bonds.
- Bubbles and circles are not particles of matter. Bubbles are formed by the arrangement of molecules in a liquid or solid, and circles are geometric shapes with no physical substance.
- Therefore, the correct answer is C: Atoms.
Which state of matter has particles able to slide past each other, yet still packedtogether?- a)Solids
- b)Liquids
- c)Gases
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Which state of matter has particles able to slide past each other, yet still packedtogether?
a)
Solids
b)
Liquids
c)
Gases
d)
None of the above
|
|
Stoneridge Institute answered |
Explanation:
The state of matter that has particles able to slide past each other, yet still packed together is liquids. Here's a detailed explanation:
Properties of Liquids:
- Liquids have a definite volume but not a definite shape, meaning they take the shape of their containers.
- The particles in a liquid are close together and have some degree of attraction between them, allowing them to stay packed together.
- The particles in a liquid have enough energy to move and slide past each other, which gives liquids their ability to flow.
Comparison with Other States of Matter:
- Solids: In solids, the particles are tightly packed together and have a fixed arrangement, but they do not have the ability to slide past each other like in liquids.
- Gases: In gases, the particles are much farther apart and have a lot of freedom to move. They can move in any direction and are not packed together like in liquids.
Summary:
In conclusion, liquids are the state of matter where particles are able to slide past each other while still being packed together. This property allows liquids to flow and take the shape of their containers.
The state of matter that has particles able to slide past each other, yet still packed together is liquids. Here's a detailed explanation:
Properties of Liquids:
- Liquids have a definite volume but not a definite shape, meaning they take the shape of their containers.
- The particles in a liquid are close together and have some degree of attraction between them, allowing them to stay packed together.
- The particles in a liquid have enough energy to move and slide past each other, which gives liquids their ability to flow.
Comparison with Other States of Matter:
- Solids: In solids, the particles are tightly packed together and have a fixed arrangement, but they do not have the ability to slide past each other like in liquids.
- Gases: In gases, the particles are much farther apart and have a lot of freedom to move. They can move in any direction and are not packed together like in liquids.
Summary:
In conclusion, liquids are the state of matter where particles are able to slide past each other while still being packed together. This property allows liquids to flow and take the shape of their containers.
Diwali is a famous festival of India. In this festival people burn crackers. When these crackers burn, they turn into ashes. What is such burning of crackers an example of?- a)Chemical change
- b)Physical change
- c)Both physical and chemical change
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Diwali is a famous festival of India. In this festival people burn crackers. When these crackers burn, they turn into ashes. What is such burning of crackers an example of?
a)
Chemical change
b)
Physical change
c)
Both physical and chemical change
d)
None of the above
|
|
Ameya Kapoor answered |
**Chemical Change**
Burning crackers during Diwali is an example of a chemical change. Let's understand why:
**Definition of Chemical Change:**
A chemical change, also known as a chemical reaction, is a process that results in the formation of new substances with different properties from the original substances involved. During a chemical change, the chemical composition of the substances involved is altered, and new substances are formed.
**Explanation:**
When crackers are burned during Diwali, a series of chemical reactions take place. Let's break it down:
1. **Combustion Reaction:** When a cracker is ignited, it undergoes a combustion reaction. This reaction involves the rapid combination of a substance (in this case, the cracker) with oxygen from the air to produce heat, light, and new substances. The primary reactants involved in the combustion of crackers are the chemicals present in them, such as sulfur, potassium nitrate, and carbon.
2. **Oxidation:** The combustion reaction of crackers is an oxidation reaction because oxygen is being added to the chemicals present in the crackers. This leads to the production of new compounds, such as sulfur dioxide (SO2), carbon dioxide (CO2), water (H2O), and various other gases depending on the specific composition of the cracker.
3. **Release of Energy:** During the combustion reaction, a significant amount of energy is released in the form of heat and light. This is why we see a bright display of colors and hear loud sounds when crackers are burned.
4. **Irreversibility:** Once the crackers are burned, it is not possible to reverse the chemical changes that have occurred. The original substances are transformed into new substances, and there is no simple way to revert them back to their original form.
Based on the above explanation, it is clear that the burning of crackers during Diwali involves chemical changes. Therefore, option 'A' - Chemical change is the correct answer.
Burning crackers during Diwali is an example of a chemical change. Let's understand why:
**Definition of Chemical Change:**
A chemical change, also known as a chemical reaction, is a process that results in the formation of new substances with different properties from the original substances involved. During a chemical change, the chemical composition of the substances involved is altered, and new substances are formed.
**Explanation:**
When crackers are burned during Diwali, a series of chemical reactions take place. Let's break it down:
1. **Combustion Reaction:** When a cracker is ignited, it undergoes a combustion reaction. This reaction involves the rapid combination of a substance (in this case, the cracker) with oxygen from the air to produce heat, light, and new substances. The primary reactants involved in the combustion of crackers are the chemicals present in them, such as sulfur, potassium nitrate, and carbon.
2. **Oxidation:** The combustion reaction of crackers is an oxidation reaction because oxygen is being added to the chemicals present in the crackers. This leads to the production of new compounds, such as sulfur dioxide (SO2), carbon dioxide (CO2), water (H2O), and various other gases depending on the specific composition of the cracker.
3. **Release of Energy:** During the combustion reaction, a significant amount of energy is released in the form of heat and light. This is why we see a bright display of colors and hear loud sounds when crackers are burned.
4. **Irreversibility:** Once the crackers are burned, it is not possible to reverse the chemical changes that have occurred. The original substances are transformed into new substances, and there is no simple way to revert them back to their original form.
Based on the above explanation, it is clear that the burning of crackers during Diwali involves chemical changes. Therefore, option 'A' - Chemical change is the correct answer.
The difference between boiling and evaporation is:- a)Evaporation occurs at one temperature only while boiling may occur at different temperatures
- b)Boiling changes the liquid into a gas while evaporation makes the liquid disappear
- c)Boiling occurs at one temperature while evaporation may occur at different temperatures
- d)There is no difference between boiling and evaporation as in each case a liquidis changed into a gas
Correct answer is option 'C'. Can you explain this answer?
The difference between boiling and evaporation is:
a)
Evaporation occurs at one temperature only while boiling may occur at different temperatures
b)
Boiling changes the liquid into a gas while evaporation makes the liquid disappear
c)
Boiling occurs at one temperature while evaporation may occur at different temperatures
d)
There is no difference between boiling and evaporation as in each case a liquidis changed into a gas
|
|
Snehal Sengupta answered |
Introduction:
Boiling and evaporation are both processes that involve the conversion of a liquid into a gas. Although they may seem similar, there are some key differences between the two.
Difference between boiling and evaporation:
1. Temperature:
- Boiling: Boiling occurs at a specific temperature known as the boiling point of the liquid. This temperature is different for different liquids. For example, water boils at 100 degrees Celsius at sea level.
- Evaporation: Evaporation, on the other hand, can occur at any temperature below the boiling point of the liquid. It is a slower process that happens gradually as the liquid particles gain enough energy to escape into the air.
2. Process:
- Boiling: Boiling is a rapid process in which bubbles of vapor form within the liquid and rise to the surface. This happens when the temperature of the liquid reaches its boiling point.
- Evaporation: Evaporation occurs when the liquid particles at the surface gain enough kinetic energy to escape into the air as vapor. It happens at the surface of the liquid and does not involve the formation of bubbles.
3. Heat Source:
- Boiling: Boiling requires the addition of heat to the liquid from an external source, such as a stove or a flame. The heat causes the liquid to reach its boiling point and undergo a phase change.
- Evaporation: Evaporation can occur without the addition of external heat. It happens naturally as the liquid particles gain energy from their surroundings, such as sunlight or room temperature.
4. Occurrence:
- Boiling: Boiling occurs throughout the liquid, as the heat is evenly distributed and reaches all parts of the liquid. This is why bubbles form throughout the liquid during boiling.
- Evaporation: Evaporation occurs only at the surface of the liquid. The liquid gradually reduces in volume as the surface particles escape as vapor, but the bulk of the liquid remains unaffected.
Summary:
In summary, the main difference between boiling and evaporation is that boiling occurs at a specific temperature (boiling point) and requires the addition of external heat, while evaporation can occur at any temperature below the boiling point and does not necessarily require external heat. Boiling is a rapid process that involves the formation of bubbles throughout the liquid, while evaporation is a slower process that occurs only at the surface of the liquid.
Boiling and evaporation are both processes that involve the conversion of a liquid into a gas. Although they may seem similar, there are some key differences between the two.
Difference between boiling and evaporation:
1. Temperature:
- Boiling: Boiling occurs at a specific temperature known as the boiling point of the liquid. This temperature is different for different liquids. For example, water boils at 100 degrees Celsius at sea level.
- Evaporation: Evaporation, on the other hand, can occur at any temperature below the boiling point of the liquid. It is a slower process that happens gradually as the liquid particles gain enough energy to escape into the air.
2. Process:
- Boiling: Boiling is a rapid process in which bubbles of vapor form within the liquid and rise to the surface. This happens when the temperature of the liquid reaches its boiling point.
- Evaporation: Evaporation occurs when the liquid particles at the surface gain enough kinetic energy to escape into the air as vapor. It happens at the surface of the liquid and does not involve the formation of bubbles.
3. Heat Source:
- Boiling: Boiling requires the addition of heat to the liquid from an external source, such as a stove or a flame. The heat causes the liquid to reach its boiling point and undergo a phase change.
- Evaporation: Evaporation can occur without the addition of external heat. It happens naturally as the liquid particles gain energy from their surroundings, such as sunlight or room temperature.
4. Occurrence:
- Boiling: Boiling occurs throughout the liquid, as the heat is evenly distributed and reaches all parts of the liquid. This is why bubbles form throughout the liquid during boiling.
- Evaporation: Evaporation occurs only at the surface of the liquid. The liquid gradually reduces in volume as the surface particles escape as vapor, but the bulk of the liquid remains unaffected.
Summary:
In summary, the main difference between boiling and evaporation is that boiling occurs at a specific temperature (boiling point) and requires the addition of external heat, while evaporation can occur at any temperature below the boiling point and does not necessarily require external heat. Boiling is a rapid process that involves the formation of bubbles throughout the liquid, while evaporation is a slower process that occurs only at the surface of the liquid.
Opposite of freezing is ________.- a)Sublimation
- b)vaporization
- c)Condensation
- d)melting
Correct answer is option 'D'. Can you explain this answer?
Opposite of freezing is ________.
a)
Sublimation
b)
vaporization
c)
Condensation
d)
melting
|
|
Stoneridge Institute answered |
Freezing is when liquid changes to solid, whereas melting is the change of state from solid to liquid.
As the volume of a fixed amount of gas at constant temperature decreases, its pressure .- a)Increases
- b)Decreases
- c)Stay the same
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
As the volume of a fixed amount of gas at constant temperature decreases, its pressure .
a)
Increases
b)
Decreases
c)
Stay the same
d)
None of the above
|
|
Yashina Kapoor answered |
Explanation:
When the volume of a fixed amount of gas at constant temperature decreases, its pressure increases. This relationship is described by Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume, when the temperature and amount of gas remain constant.
Here is a detailed explanation of why this occurs:
- Boyle's Law: According to Boyle's Law, the pressure of a gas is inversely proportional to its volume, when the temperature and amount of gas remain constant. This can be represented by the equation: P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
- Decreasing Volume: When the volume of a gas decreases, the same amount of gas is now confined to a smaller space. This means that the gas particles are more crowded together, resulting in more frequent collisions between the particles and the container walls.
- Increased Collisions: With more frequent collisions, there is an increase in the force exerted by the gas particles on the container walls. This increase in force leads to an increase in pressure.
- Pressure-Volume Relationship: The pressure of a gas is directly related to the force exerted by the gas particles on the container walls. As the force increases, the pressure also increases. Therefore, when the volume decreases, the pressure increases.
In conclusion, as the volume of a fixed amount of gas at constant temperature decreases, its pressure increases. This relationship is explained by Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume, when the temperature and amount of gas remain constant.
When the volume of a fixed amount of gas at constant temperature decreases, its pressure increases. This relationship is described by Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume, when the temperature and amount of gas remain constant.
Here is a detailed explanation of why this occurs:
- Boyle's Law: According to Boyle's Law, the pressure of a gas is inversely proportional to its volume, when the temperature and amount of gas remain constant. This can be represented by the equation: P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
- Decreasing Volume: When the volume of a gas decreases, the same amount of gas is now confined to a smaller space. This means that the gas particles are more crowded together, resulting in more frequent collisions between the particles and the container walls.
- Increased Collisions: With more frequent collisions, there is an increase in the force exerted by the gas particles on the container walls. This increase in force leads to an increase in pressure.
- Pressure-Volume Relationship: The pressure of a gas is directly related to the force exerted by the gas particles on the container walls. As the force increases, the pressure also increases. Therefore, when the volume decreases, the pressure increases.
In conclusion, as the volume of a fixed amount of gas at constant temperature decreases, its pressure increases. This relationship is explained by Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume, when the temperature and amount of gas remain constant.
What determines the speed of the atoms and molecules of a particular substance?- a)Size of the atoms and molecules
- b)Temperature of the substance
- c)None of the above
- d)Both A and B
Correct answer is option 'D'. Can you explain this answer?
What determines the speed of the atoms and molecules of a particular substance?
a)
Size of the atoms and molecules
b)
Temperature of the substance
c)
None of the above
d)
Both A and B
|
|
Yashina Kapoor answered |
Factors determining the speed of atoms and molecules in a substance:
The speed of atoms and molecules in a substance is determined by both the size of the atoms and molecules and the temperature of the substance. Here's a detailed explanation:
Size of the atoms and molecules:
- The size of atoms and molecules plays a role in determining their speed.
- Smaller atoms and molecules tend to move faster than larger ones due to their lighter mass and higher kinetic energy.
- The speed of atoms and molecules can be influenced by factors such as atomic or molecular weight and atomic or molecular radius.
Temperature of the substance:
- Temperature is a measure of the average kinetic energy of atoms and molecules in a substance.
- When the temperature of a substance increases, the average kinetic energy and speed of the atoms and molecules also increase.
- This is because an increase in temperature leads to an increase in the vibrational, rotational, and translational motion of the atoms and molecules.
- Conversely, a decrease in temperature results in a decrease in the speed of the atoms and molecules.
Interaction between size and temperature:
- The speed of atoms and molecules is influenced by both the size and temperature.
- At a given temperature, smaller atoms and molecules will generally have higher speeds than larger ones.
- However, at the same size, an increase in temperature will cause all atoms and molecules to move faster.
Therefore, the speed of atoms and molecules in a particular substance is determined by both the size of the atoms and molecules and the temperature of the substance.
The speed of atoms and molecules in a substance is determined by both the size of the atoms and molecules and the temperature of the substance. Here's a detailed explanation:
Size of the atoms and molecules:
- The size of atoms and molecules plays a role in determining their speed.
- Smaller atoms and molecules tend to move faster than larger ones due to their lighter mass and higher kinetic energy.
- The speed of atoms and molecules can be influenced by factors such as atomic or molecular weight and atomic or molecular radius.
Temperature of the substance:
- Temperature is a measure of the average kinetic energy of atoms and molecules in a substance.
- When the temperature of a substance increases, the average kinetic energy and speed of the atoms and molecules also increase.
- This is because an increase in temperature leads to an increase in the vibrational, rotational, and translational motion of the atoms and molecules.
- Conversely, a decrease in temperature results in a decrease in the speed of the atoms and molecules.
Interaction between size and temperature:
- The speed of atoms and molecules is influenced by both the size and temperature.
- At a given temperature, smaller atoms and molecules will generally have higher speeds than larger ones.
- However, at the same size, an increase in temperature will cause all atoms and molecules to move faster.
Therefore, the speed of atoms and molecules in a particular substance is determined by both the size of the atoms and molecules and the temperature of the substance.
Gases consist of particles that:- a)Are packed close together
- b)Are strongly attracted to each other
- c)Have a regular arrangement
- d)Are very far apart
Correct answer is option 'D'. Can you explain this answer?
Gases consist of particles that:
a)
Are packed close together
b)
Are strongly attracted to each other
c)
Have a regular arrangement
d)
Are very far apart
|
|
Edgy Education answered |
Explanation:
To answer this question, we need to understand the characteristics of gases and their particles. Gases are one of the three common states of matter, along with solids and liquids. Unlike solids and liquids, gases have particles that are very far apart from each other and have no fixed arrangement. Let's break it down further:
1. Are packed close together:
- This is not true for gases. In gases, the particles are not packed closely together like in solids or liquids.
2. Are strongly attracted to each other:
- Gases do not have strong attractive forces between their particles. Unlike solids or liquids, the intermolecular forces in gases are weak.
3. Have a regular arrangement:
- Gases do not have a regular arrangement. The particles move randomly and freely in all directions.
4. Are very far apart:
- This is the correct answer. Gases consist of particles that are very far apart from each other. The distance between gas particles is much larger compared to the distance between particles in solids or liquids.
In conclusion, gases consist of particles that are very far apart from each other. They do not have a regular arrangement and are not strongly attracted to each other.
To answer this question, we need to understand the characteristics of gases and their particles. Gases are one of the three common states of matter, along with solids and liquids. Unlike solids and liquids, gases have particles that are very far apart from each other and have no fixed arrangement. Let's break it down further:
1. Are packed close together:
- This is not true for gases. In gases, the particles are not packed closely together like in solids or liquids.
2. Are strongly attracted to each other:
- Gases do not have strong attractive forces between their particles. Unlike solids or liquids, the intermolecular forces in gases are weak.
3. Have a regular arrangement:
- Gases do not have a regular arrangement. The particles move randomly and freely in all directions.
4. Are very far apart:
- This is the correct answer. Gases consist of particles that are very far apart from each other. The distance between gas particles is much larger compared to the distance between particles in solids or liquids.
In conclusion, gases consist of particles that are very far apart from each other. They do not have a regular arrangement and are not strongly attracted to each other.
When heat is supplied to molecules, their ___________ energy increases.- a)Chemical
- b)Kinetic
- c)Potential
- d)Electrical
Correct answer is option 'B'. Can you explain this answer?
When heat is supplied to molecules, their ___________ energy increases.
a)
Chemical
b)
Kinetic
c)
Potential
d)
Electrical
|
|
Aman Desai answered |
Answer:
Explanation:
When heat is supplied to molecules, their kinetic energy increases.
Kinetic Energy:
- Kinetic energy refers to the energy of motion.
- It is the energy possessed by an object due to its motion.
- The faster an object moves, the more kinetic energy it has.
Heat and Kinetic Energy:
- Heat is a form of energy that can be transferred from one object to another due to a temperature difference.
- When heat is added to a substance, its internal energy increases.
- This increase in internal energy leads to an increase in the kinetic energy of the molecules.
Effect of Heat on Molecules:
- Molecules are constantly in motion, vibrating and moving around.
- When heat is supplied to molecules, it causes an increase in their kinetic energy.
- This increase in kinetic energy results in an increase in the speed and motion of the molecules.
- As the molecules move faster, they collide more frequently and with greater force.
Examples:
- Consider a solid substance, such as ice. When heat is applied to the ice, its molecules gain kinetic energy and start moving faster.
- As the kinetic energy increases, the ice melts into liquid water, where the molecules have even higher kinetic energy.
- If more heat is supplied, the water molecules gain even more kinetic energy and eventually turn into water vapor, which is a gas.
Conclusion:
When heat is supplied to molecules, their kinetic energy increases. This increase in kinetic energy leads to an increase in the speed and motion of the molecules.
Explanation:
When heat is supplied to molecules, their kinetic energy increases.
Kinetic Energy:
- Kinetic energy refers to the energy of motion.
- It is the energy possessed by an object due to its motion.
- The faster an object moves, the more kinetic energy it has.
Heat and Kinetic Energy:
- Heat is a form of energy that can be transferred from one object to another due to a temperature difference.
- When heat is added to a substance, its internal energy increases.
- This increase in internal energy leads to an increase in the kinetic energy of the molecules.
Effect of Heat on Molecules:
- Molecules are constantly in motion, vibrating and moving around.
- When heat is supplied to molecules, it causes an increase in their kinetic energy.
- This increase in kinetic energy results in an increase in the speed and motion of the molecules.
- As the molecules move faster, they collide more frequently and with greater force.
Examples:
- Consider a solid substance, such as ice. When heat is applied to the ice, its molecules gain kinetic energy and start moving faster.
- As the kinetic energy increases, the ice melts into liquid water, where the molecules have even higher kinetic energy.
- If more heat is supplied, the water molecules gain even more kinetic energy and eventually turn into water vapor, which is a gas.
Conclusion:
When heat is supplied to molecules, their kinetic energy increases. This increase in kinetic energy leads to an increase in the speed and motion of the molecules.
Pick the correct option.- a)Solids can freeze
- b)Liquids can freeze
- c)Gases can freeze
- d)All of the above
Correct answer is option 'B'. Can you explain this answer?
Pick the correct option.
a)
Solids can freeze
b)
Liquids can freeze
c)
Gases can freeze
d)
All of the above
|
|
Sagarika Kulkarni answered |
Explanation:
Solids can freeze:
- Solids are one of the three states of matter, along with liquids and gases.
- Freezing is the process in which a substance changes from its liquid state to its solid state.
- When a solid freezes, the particles in the substance slow down and come closer together, forming a rigid structure.
- This can be observed in everyday life when water freezes and turns into ice.
Liquids can freeze:
- Liquids are another state of matter, and they can also undergo the process of freezing.
- When a liquid is cooled, its particles slow down and come together, forming a solid.
- For example, when water is cooled below its freezing point (0 degrees Celsius or 32 degrees Fahrenheit), it freezes and becomes ice.
Gases cannot freeze:
- Gases, on the other hand, do not freeze in the same way as solids and liquids.
- Freezing is the process of changing from a liquid to a solid state, and gases do not have a liquid state.
- Instead, gases can undergo a process called condensation, where they change from a gaseous state to a liquid state when cooled.
- However, it is important to note that gases can be converted into a solid directly through a process called deposition, skipping the liquid state.
Conclusion:
- Based on the above explanations, the correct option is 'b) Liquids can freeze'.
- Solids can also freeze, but it is not the only correct option.
- Gases cannot freeze, but they can undergo condensation or deposition to change state.
Solids can freeze:
- Solids are one of the three states of matter, along with liquids and gases.
- Freezing is the process in which a substance changes from its liquid state to its solid state.
- When a solid freezes, the particles in the substance slow down and come closer together, forming a rigid structure.
- This can be observed in everyday life when water freezes and turns into ice.
Liquids can freeze:
- Liquids are another state of matter, and they can also undergo the process of freezing.
- When a liquid is cooled, its particles slow down and come together, forming a solid.
- For example, when water is cooled below its freezing point (0 degrees Celsius or 32 degrees Fahrenheit), it freezes and becomes ice.
Gases cannot freeze:
- Gases, on the other hand, do not freeze in the same way as solids and liquids.
- Freezing is the process of changing from a liquid to a solid state, and gases do not have a liquid state.
- Instead, gases can undergo a process called condensation, where they change from a gaseous state to a liquid state when cooled.
- However, it is important to note that gases can be converted into a solid directly through a process called deposition, skipping the liquid state.
Conclusion:
- Based on the above explanations, the correct option is 'b) Liquids can freeze'.
- Solids can also freeze, but it is not the only correct option.
- Gases cannot freeze, but they can undergo condensation or deposition to change state.
Which of the followings is a physical change?- a)Changing of wheat to bread
- b)Rusting of iron
- c)Melting of butter
- d)Burning of paper
Correct answer is option 'C'. Can you explain this answer?
Which of the followings is a physical change?
a)
Changing of wheat to bread
b)
Rusting of iron
c)
Melting of butter
d)
Burning of paper
|
|
Ashish Malik answered |
Melting of butter is a physical change.
Explanation:
A physical change is a change in the physical properties of a substance, such as its shape, size, or state, without changing its chemical composition. In the case of melting butter, the butter changes from a solid state to a liquid state without undergoing any chemical reactions.
Here is a detailed explanation of why the other options are not physical changes:
a) Changing of wheat to bread: This is a chemical change because the wheat undergoes a process called baking, where it combines with other ingredients and undergoes a chemical reaction to form bread. The composition of the wheat changes, so it is not a physical change.
b) Rusting of iron: Rusting is a chemical change. When iron reacts with oxygen and moisture in the air, it forms iron oxide (rust). This is a chemical reaction that changes the composition of the iron, so it is not a physical change.
c) Melting of butter: This is a physical change. Butter is a solid at room temperature, but when heated, it melts and becomes a liquid. The molecules in the butter rearrange themselves, but the chemical composition of the butter remains the same.
d) Burning of paper: Burning is a chemical change. When paper burns, it undergoes a combustion reaction where it reacts with oxygen in the air and releases heat, light, and gases. The original paper is converted into ash, gases, and smoke, so it is not a physical change.
In summary, the melting of butter is a physical change because it only involves a change in the physical state of the substance without altering its chemical composition. The other options involve chemical changes where the composition of the substances changes.
Explanation:
A physical change is a change in the physical properties of a substance, such as its shape, size, or state, without changing its chemical composition. In the case of melting butter, the butter changes from a solid state to a liquid state without undergoing any chemical reactions.
Here is a detailed explanation of why the other options are not physical changes:
a) Changing of wheat to bread: This is a chemical change because the wheat undergoes a process called baking, where it combines with other ingredients and undergoes a chemical reaction to form bread. The composition of the wheat changes, so it is not a physical change.
b) Rusting of iron: Rusting is a chemical change. When iron reacts with oxygen and moisture in the air, it forms iron oxide (rust). This is a chemical reaction that changes the composition of the iron, so it is not a physical change.
c) Melting of butter: This is a physical change. Butter is a solid at room temperature, but when heated, it melts and becomes a liquid. The molecules in the butter rearrange themselves, but the chemical composition of the butter remains the same.
d) Burning of paper: Burning is a chemical change. When paper burns, it undergoes a combustion reaction where it reacts with oxygen in the air and releases heat, light, and gases. The original paper is converted into ash, gases, and smoke, so it is not a physical change.
In summary, the melting of butter is a physical change because it only involves a change in the physical state of the substance without altering its chemical composition. The other options involve chemical changes where the composition of the substances changes.
Directions: Fill up the grid by answering the following questions. (Down 3) Which process is depicted in the following figure? _____________
(Down 3) Which process is depicted in the following figure? _____________
- a)Sublimation
- b)Evaporation
- c)Condensation
- d)Boiling
Correct answer is option 'B'. Can you explain this answer?
Directions: Fill up the grid by answering the following questions.

(Down 3) Which process is depicted in the following figure? _____________

a)
Sublimation
b)
Evaporation
c)
Condensation
d)
Boiling

|
Gunjan Lakhani answered |
In the given scenario, the process shown is evaporation. This occurs when:
- Heat from the sun warms the water.
- Water changes from a liquid to water vapor.
Key characteristics of evaporation include:
- It happens at the surface of the liquid.
- It does not require the liquid to reach its boiling point.
- It differs from boiling, which occurs throughout the liquid at a specific temperature.
Which change of state occurs when particles in a solid begin to move slowly past each other?
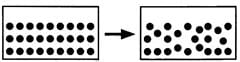
- a)Melting
- b)Boiling
- c)Condensing
- d)Subliming
Correct answer is option 'A'. Can you explain this answer?
Which change of state occurs when particles in a solid begin to move slowly past each other?


a)
Melting
b)
Boiling
c)
Condensing
d)
Subliming

|
Gunjan Lakhani answered |
The diagram has two boxes, one in which molecules are tightly packed and the other one in which the molecules are slightly loosely packed. Here solid is slowly converting to liquid state which occurs during melting. While, boiling is the process where a liquid turns into a gas. In this state change, particles gain enough energy to move freely and spread out, which does not match the situation described in the question.
Which of the following is not a form of energy?- a)Light
- b)Electricity
- c)Heat
- d)Temperature
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a form of energy?
a)
Light
b)
Electricity
c)
Heat
d)
Temperature

|
Learning Enablers answered |
Introduction:
In this question, we are asked to identify which option is not a form of energy out of the given choices.
Options:
A: Light
B: Electricity
C: Heat
D: Temperature
Explanation:
To determine which option is not a form of energy, let's analyze each option:
1. Light:
- Light is a form of energy that can be seen and detected by our eyes.
- It is a type of electromagnetic radiation and is made up of particles called photons.
2. Electricity:
- Electricity is a form of energy resulting from the flow of electrons or electric charge.
- It is commonly used to power various devices and is a fundamental form of energy in modern society.
3. Heat:
- Heat is a form of energy that results from the transfer of thermal energy between objects.
- It can be measured in terms of temperature and is associated with the motion of particles.
4. Temperature:
- Temperature is not a form of energy but rather a measure of the average kinetic energy of particles in a substance.
- It is a physical property that indicates the hotness or coldness of an object or environment.
Conclusion:
Out of the given options, Temperature is not a form of energy. It is a measure of the average kinetic energy of particles and not an energy form itself.
Which of the following is a physical change?- a)Ripening of mango
- b)Baking of cake
- c)Breaking of glass
- d)Melting of ice
Correct answer is option 'D'. Can you explain this answer?
a)
Ripening of mango
b)
Baking of cake
c)
Breaking of glass
d)
Melting of ice
|
|
Stoneridge Institute answered |
The melting of ice is a physical change. It involves a transition from solid to liquid without changing the chemical composition of the substance.
- When ice melts, it simply changes state.
- No new substances are formed during this process.
- This distinguishes it from chemical changes, where the identity of the substance changes.
Melting is the opposite of:- a)Vapourization
- b)Freezing
- c)Sublimation
- d)Condensation
Correct answer is option 'B'. Can you explain this answer?
Melting is the opposite of:
a)
Vapourization
b)
Freezing
c)
Sublimation
d)
Condensation
|
|
Edgy Education answered |
To answer this question, we need to understand the process of melting and its opposite.
Melting:
- Melting is a physical process in which a substance changes from a solid state to a liquid state.
- It occurs when the temperature of the substance reaches its melting point.
- During melting, the substance absorbs heat energy, which breaks the intermolecular forces holding the particles together, allowing them to move freely.
The Opposite of Melting:
The opposite of melting is the process in which a substance changes from a liquid state to a solid state. This process is known as freezing.
Freezing:
- Freezing is a physical process in which a substance changes from a liquid state to a solid state.
- It occurs when the temperature of the substance reaches its freezing point.
- During freezing, the substance releases heat energy, causing the particles to slow down and form a regular crystalline structure.
Answer:
The opposite of melting is freezing (option B).
The diagram given below depicts which of the processes of the change of state of matter?

- a)Sublimation
- b)Evaporation
- c)Vaporization
- d)Condensation
Correct answer is option 'D'. Can you explain this answer?
The diagram given below depicts which of the processes of the change of state of matter?


a)
Sublimation
b)
Evaporation
c)
Vaporization
d)
Condensation
|
|
Edu Impact answered |
As seen in the diagram, there are small water drops on the window. These water drops can be seen as a result of the conversion of water vapour in the air back into the water form. Thus this depicts the process of condensation.
Which of the following can be compressed?P: steam
Q: oxygen
R: carbon dioxide- a)Only P and Q
- b)Only P and R
- c)Only Q and R
- d)P, Q and R
Correct answer is option 'D'. Can you explain this answer?
Which of the following can be compressed?
P: steam
Q: oxygen
R: carbon dioxide
Q: oxygen
R: carbon dioxide
a)
Only P and Q
b)
Only P and R
c)
Only Q and R
d)
P, Q and R

|
Gunjan Lakhani answered |
All three, steam, oxygen, and carbon dioxide, can be compressed.
- Steam is a gas and, like any gas, can be compressed by reducing the volume and increasing the pressure.
- Oxygen is a gas and can also be compressed under pressure.
- Carbon dioxide is a gas and can be compressed into a liquid or solid form under high pressure.
Therefore, the correct answer is Option D (P, Q and R).
As the temperature of a fixed amount of gas at constant volume decreases, itspressure .- a)Increases
- b)Decreases
- c)Stay the same
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
As the temperature of a fixed amount of gas at constant volume decreases, itspressure .
a)
Increases
b)
Decreases
c)
Stay the same
d)
None of the above
|
|
Deepika Tiwari answered |
Explanation:
When the temperature of a fixed amount of gas at constant volume decreases, its pressure decreases. This is explained by the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature, assuming constant volume and amount of gas. The ideal gas law is represented by the equation:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of gas
- R is the ideal gas constant
- T is the temperature of the gas
When the temperature of a gas decreases while keeping the volume constant, according to the ideal gas law, the pressure of the gas must also decrease. This can be understood by examining the relationship between temperature and pressure in the ideal gas law equation.
Key Points:
- The ideal gas law states that the pressure of a gas is directly proportional to its temperature, assuming constant volume and amount of gas.
- When the temperature of a gas decreases while the volume is kept constant, the pressure of the gas also decreases.
- This is because the decrease in temperature leads to a decrease in the average kinetic energy of the gas particles.
- With lower kinetic energy, the gas particles collide with the walls of the container less frequently and with less force, resulting in a decrease in pressure.
- Conversely, when the temperature of a gas increases, the pressure of the gas also increases, as the gas particles have higher kinetic energy and collide with the walls of the container more frequently and with greater force.
Conclusion:
In summary, when the temperature of a fixed amount of gas at constant volume decreases, its pressure decreases. This is due to the decrease in the average kinetic energy of the gas particles, resulting in fewer and weaker collisions with the walls of the container.
When the temperature of a fixed amount of gas at constant volume decreases, its pressure decreases. This is explained by the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature, assuming constant volume and amount of gas. The ideal gas law is represented by the equation:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of gas
- R is the ideal gas constant
- T is the temperature of the gas
When the temperature of a gas decreases while keeping the volume constant, according to the ideal gas law, the pressure of the gas must also decrease. This can be understood by examining the relationship between temperature and pressure in the ideal gas law equation.
Key Points:
- The ideal gas law states that the pressure of a gas is directly proportional to its temperature, assuming constant volume and amount of gas.
- When the temperature of a gas decreases while the volume is kept constant, the pressure of the gas also decreases.
- This is because the decrease in temperature leads to a decrease in the average kinetic energy of the gas particles.
- With lower kinetic energy, the gas particles collide with the walls of the container less frequently and with less force, resulting in a decrease in pressure.
- Conversely, when the temperature of a gas increases, the pressure of the gas also increases, as the gas particles have higher kinetic energy and collide with the walls of the container more frequently and with greater force.
Conclusion:
In summary, when the temperature of a fixed amount of gas at constant volume decreases, its pressure decreases. This is due to the decrease in the average kinetic energy of the gas particles, resulting in fewer and weaker collisions with the walls of the container.
Water vapor and steam are similar because they- a)have no definite shape and no definite volume.
- b)have definite shape and no definite volume.
- c)have no definite shape but definite volume.
- d)have definite mass and definite volume.
Correct answer is option 'A'. Can you explain this answer?
a)
have no definite shape and no definite volume.
b)
have definite shape and no definite volume.
c)
have no definite shape but definite volume.
d)
have definite mass and definite volume.

|
Indu Gupta answered |
Both water vapor and steam are classified as gases. They share the following characteristics:
- They have no definite shape.
- They have no definite volume.
- They expand to fill any container.
While steam is the gaseous form of water produced at boiling point, water vapor refers to water in its gaseous state below boiling. In essence, both exhibit the typical properties of gases.
There is a gold chain weighing 100 g. If the jeweller melts 100 g of pure gold andsells it, he will get:- a)Price for 100 g of gold
- b)Price for 80 g of gold
- c)Price for 130 g of gold
- d)Price for 10 g of gold
Correct answer is option 'A'. Can you explain this answer?
There is a gold chain weighing 100 g. If the jeweller melts 100 g of pure gold andsells it, he will get:
a)
Price for 100 g of gold
b)
Price for 80 g of gold
c)
Price for 130 g of gold
d)
Price for 10 g of gold
|
|
Vaishnavi Sharma answered |
Answer:
Given:
- Weight of gold chain = 100 g
- Weight of pure gold melted and sold = 100 g
To find out:
- Price for 100 g of gold
Solution:
Gold is a precious metal and its price keeps fluctuating in the market. The price of gold is usually measured in terms of its weight and purity.
Price for 100 g of gold:
- As per the given information, the jeweller melted and sold 100 g of pure gold.
- The price of gold varies in the market, but let's assume that the current price of gold is $50 per gram.
- Therefore, the price for 100 g of gold would be:
100 g * $50/g = $5000
Price for 80 g of gold:
- If the jeweller had melted and sold only 80 g of pure gold, then the price would be:
80 g * $50/g = $4000
Price for 130 g of gold:
- This option is not applicable as the jeweller had only melted and sold 100 g of pure gold.
Price for 10 g of gold:
- If the jeweller had melted and sold only 10 g of pure gold, then the price would be:
10 g * $50/g = $500
Therefore, the correct answer is option 'A', which is the price for 100 g of gold.
Given:
- Weight of gold chain = 100 g
- Weight of pure gold melted and sold = 100 g
To find out:
- Price for 100 g of gold
Solution:
Gold is a precious metal and its price keeps fluctuating in the market. The price of gold is usually measured in terms of its weight and purity.
Price for 100 g of gold:
- As per the given information, the jeweller melted and sold 100 g of pure gold.
- The price of gold varies in the market, but let's assume that the current price of gold is $50 per gram.
- Therefore, the price for 100 g of gold would be:
100 g * $50/g = $5000
Price for 80 g of gold:
- If the jeweller had melted and sold only 80 g of pure gold, then the price would be:
80 g * $50/g = $4000
Price for 130 g of gold:
- This option is not applicable as the jeweller had only melted and sold 100 g of pure gold.
Price for 10 g of gold:
- If the jeweller had melted and sold only 10 g of pure gold, then the price would be:
10 g * $50/g = $500
Therefore, the correct answer is option 'A', which is the price for 100 g of gold.
What makes liquid flow?- a)In liquids, particles are tightly packed,
- b)In liquids, particles are packed in a rigid pattern.
- c)In liquids, particles are not tightly packed and can slide.
- d)All of these
Correct answer is option 'C'. Can you explain this answer?
What makes liquid flow?
a)
In liquids, particles are tightly packed,
b)
In liquids, particles are packed in a rigid pattern.
c)
In liquids, particles are not tightly packed and can slide.
d)
All of these

|
Dr Manju Sen answered |
The ability of liquids to flow is due to the fact that their particles are not tightly packed like in solids. The particles have enough space between them to move or slide past each other, allowing the liquid to flow and take the shape of its container. This is a key characteristic of liquids.
Which of the following is true for the molecules of gases?- a)They can move around freely
- b)They have a fixed volume
- c)They have a fixed shape
- d)They cannot move freely
Correct answer is option 'A'. Can you explain this answer?
Which of the following is true for the molecules of gases?
a)
They can move around freely
b)
They have a fixed volume
c)
They have a fixed shape
d)
They cannot move freely
|
|
Ameya Kapoor answered |
Gas molecules can move around freely.
Gas molecules are characterized by their high kinetic energy and lack of intermolecular forces. This allows them to move around freely in all directions within the container they are in. This is in contrast to the molecules of liquids and solids, which have stronger intermolecular forces that restrict their movement.
Explanation:
Gas molecules are in constant motion due to their high kinetic energy. They move randomly and collide with each other and the walls of their container. This random motion is known as Brownian motion.
Key Points:
- Gas molecules have a high kinetic energy and are in constant motion.
- They move randomly in all directions.
- Gas molecules collide with each other and the walls of their container.
- This random motion is known as Brownian motion.
- The lack of intermolecular forces in gases allows the molecules to move around freely.
Example:
To understand this concept, let's consider a balloon filled with air. The air inside the balloon is made up of gas molecules. When the balloon is squeezed or released, the gas molecules inside move in response to the change in pressure. They spread out when the balloon is released, moving freely in all directions. This is because there are no strong intermolecular forces holding the gas molecules together.
Conclusion:
In conclusion, gas molecules have the ability to move around freely due to their high kinetic energy and lack of intermolecular forces. This allows them to occupy the entire volume of the container they are in and explains why gases do not have a fixed shape or volume.
Gas molecules are characterized by their high kinetic energy and lack of intermolecular forces. This allows them to move around freely in all directions within the container they are in. This is in contrast to the molecules of liquids and solids, which have stronger intermolecular forces that restrict their movement.
Explanation:
Gas molecules are in constant motion due to their high kinetic energy. They move randomly and collide with each other and the walls of their container. This random motion is known as Brownian motion.
Key Points:
- Gas molecules have a high kinetic energy and are in constant motion.
- They move randomly in all directions.
- Gas molecules collide with each other and the walls of their container.
- This random motion is known as Brownian motion.
- The lack of intermolecular forces in gases allows the molecules to move around freely.
Example:
To understand this concept, let's consider a balloon filled with air. The air inside the balloon is made up of gas molecules. When the balloon is squeezed or released, the gas molecules inside move in response to the change in pressure. They spread out when the balloon is released, moving freely in all directions. This is because there are no strong intermolecular forces holding the gas molecules together.
Conclusion:
In conclusion, gas molecules have the ability to move around freely due to their high kinetic energy and lack of intermolecular forces. This allows them to occupy the entire volume of the container they are in and explains why gases do not have a fixed shape or volume.
Chapter doubts & questions for Matter and Materials - Science Olympiad for Class 5 2025 is part of Class 5 exam preparation. The chapters have been prepared according to the Class 5 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 5 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Matter and Materials - Science Olympiad for Class 5 in English & Hindi are available as part of Class 5 exam.
Download more important topics, notes, lectures and mock test series for Class 5 Exam by signing up for free.
Science Olympiad for Class 5
29 videos|73 docs|43 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









