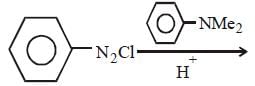
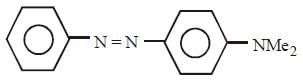
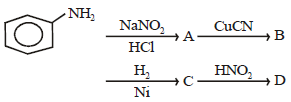
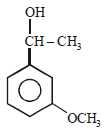
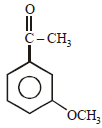
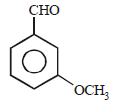
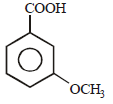
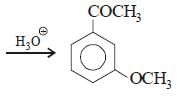
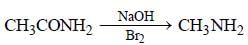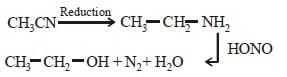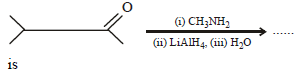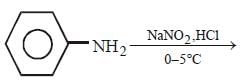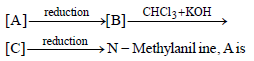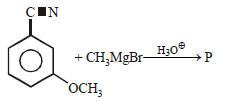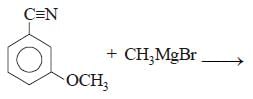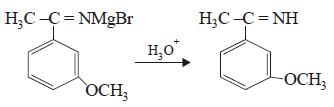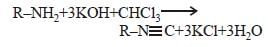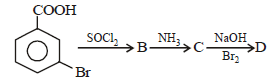All Exams >
NEET >
NEET Past Year Papers >
All Questions
All questions of Amines for NEET Exam
The consituent of the powerful explosive RDX is formed during the nitration of [2000]- a)toluene
- b)phenol
- c)glycerol
- d)urotropine
Correct answer is option 'D'. Can you explain this answer?
The consituent of the powerful explosive RDX is formed during the nitration of [2000]
a)
toluene
b)
phenol
c)
glycerol
d)
urotropine

|
Anirudh Datta answered |
RDX is prepared by treating urotropine with fuming nitric acid. When the inner bridge system is destroyed by oxidation and the peripheral nitrogen atom are nitrated, it forms cyclonitrite (or RDX).
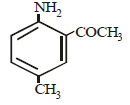
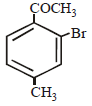
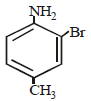
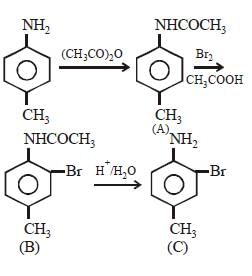
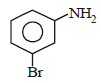
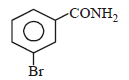
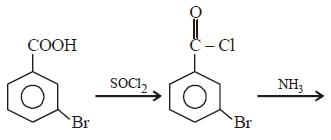
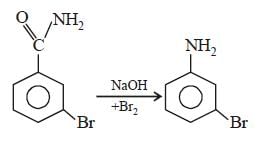
Intermediates formed during reaction of 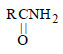 with Br2 and KOH are [2001]
with Br2 and KOH are [2001]- a)RNHBr and RCONHBr
- b)RNHCOBr and RNCO
- c)RCONHBr and RNCO
- d)RCONBr2
Correct answer is option 'C'. Can you explain this answer?
Intermediates formed during reaction of 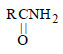 with Br2 and KOH are [2001]
with Br2 and KOH are [2001]
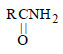 with Br2 and KOH are [2001]
with Br2 and KOH are [2001]a)
RNHBr and RCONHBr
b)
RNHCOBr and RNCO
c)
RCONHBr and RNCO
d)
RCONBr2
|
|
Pooja Shah answered |
The mecahnism of Hoffmann bromide
reaction is
reaction is
(i)
RCONH2 + Br2 →RCONHBr + HBr
(ii)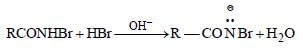

(iii)
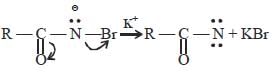
(iv)
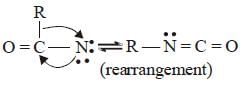
(v)
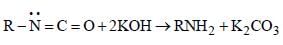
Acetamide and ethylamine can be distinguishedby reacting with [1994]- a)Aqueous HCl and heat
- b)Aqueous NaOH and heat
- c)Acidified KMnO4
- d)Bromine water.
Correct answer is option 'B'. Can you explain this answer?
Acetamide and ethylamine can be distinguishedby reacting with [1994]
a)
Aqueous HCl and heat
b)
Aqueous NaOH and heat
c)
Acidified KMnO4
d)
Bromine water.

|
Sarthak Saini answered |
Acetamide and ethylamine can be
distinguished by heating with NaOH
solution. Acetamide evolves NH3 but
ethylamine does not.
distinguished by heating with NaOH
solution. Acetamide evolves NH3 but
ethylamine does not.
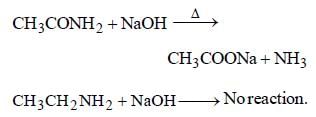
For carbylamine reaction, we need hot alcoholic KOH and [1992]- a)Any primary amine and chloroform
- b)Chloroform and silver powder
- c)A primary amine and an alkyl halide
- d)A monoalkylamine and trichloromethane.
Correct answer is option 'A'. Can you explain this answer?
For carbylamine reaction, we need hot alcoholic KOH and [1992]
a)
Any primary amine and chloroform
b)
Chloroform and silver powder
c)
A primary amine and an alkyl halide
d)
A monoalkylamine and trichloromethane.

|
Kajal Bose answered |
Any primary amine means both aliphatic as well as aromatic but onoalkylamines means only 1° aliphatic amines. Therefore, option (a) is correct while (d) is wrong.
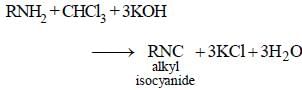
What is the decreasing order of basicity of primary, secondary and tertiary ethylamines and NH3 ? [1994]- a)NH3 > C2H5NH2 > (C2H5)2NH >(C2H5)3N
- b)(C2H5)3N > (C2H5)2NH >C2H5NH2 > NH3
- c)(C2H5)2NH > C2H5NH2 >(C2H5)3N > NH3
- d)(C2H5 )2NH > (C2H5 )3N >C2H5NH2 > NH3.
Correct answer is option 'D'. Can you explain this answer?
What is the decreasing order of basicity of primary, secondary and tertiary ethylamines and NH3 ? [1994]
a)
NH3 > C2H5NH2 > (C2H5)2NH >(C2H5)3N
b)
(C2H5)3N > (C2H5)2NH >C2H5NH2 > NH3
c)
(C2H5)2NH > C2H5NH2 >(C2H5)3N > NH3
d)
(C2H5 )2NH > (C2H5 )3N >C2H5NH2 > NH3.

|
Maya Sengupta answered |
All aliphatic amines are stronger bases than NH3 and among different ethylamines order of basictity is 2° > 3° > 1°. Thus, the correct order is (d) i.e.,
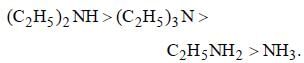
This anomolous behaviour of tertiary ethyl amine is due to steric factors i.e., crowding of alkyl groups cover nitrogen atom from all sides and thus makes the approach and bonding by a lewis acid relatively difficult which results the maximum steric strain in tertiary amines. The electrons are there but the path is blocked resulting the reduction in its basicity.
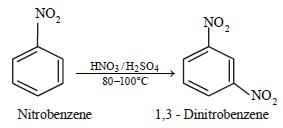
Nitrobenzene can be prepared from benzene byusing a mixture of conc. HNO3 and conc. H2SO4 in the mixture, nitric acid acts as a/an: [2009]- a)acid
- b)base
- c)catalyst
- d)reducing agent
Correct answer is option 'B'. Can you explain this answer?
Nitrobenzene can be prepared from benzene byusing a mixture of conc. HNO3 and conc. H2SO4 in the mixture, nitric acid acts as a/an: [2009]
a)
acid
b)
base
c)
catalyst
d)
reducing agent

|
Muskaan Basak answered |
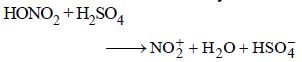
Nitric acid acts as a base by accepting a proton.
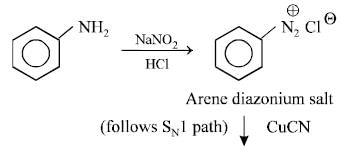
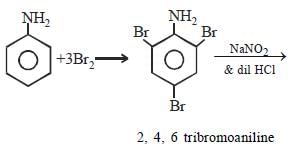
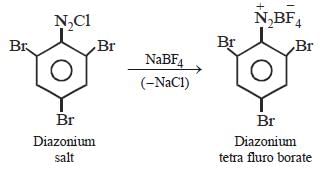
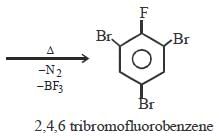
Aniline is reacted with bromine water and theresulting product is treated with an aqueoussolution of sodium nitrite in presence of dilutehydrochloric acid. The compound so formed isconverted into a tetrafluoroborate which issubsequently heated dry. The final product is [1998]- a)1,3, 5-tribromobenzene
- b)p-bromofluorobenzene
- c)p-bromoaniline
- d)2,4, 6-tribromofluorobenzene
Correct answer is option 'D'. Can you explain this answer?
Aniline is reacted with bromine water and theresulting product is treated with an aqueoussolution of sodium nitrite in presence of dilutehydrochloric acid. The compound so formed isconverted into a tetrafluoroborate which issubsequently heated dry. The final product is [1998]
a)
1,3, 5-tribromobenzene
b)
p-bromofluorobenzene
c)
p-bromoaniline
d)
2,4, 6-tribromofluorobenzene

|
Nayanika Dasgupta answered |
Which one of the following on reduction withlithium aluminium hydride yields a secondaryamine? [2007]- a)Methyl isocyanide
- b)Acetamide
- c)Methyl cyanide
- d)Nitroethane.
Correct answer is option 'A'. Can you explain this answer?
Which one of the following on reduction withlithium aluminium hydride yields a secondaryamine? [2007]
a)
Methyl isocyanide
b)
Acetamide
c)
Methyl cyanide
d)
Nitroethane.

|
Bhargavi Choudhury answered |
Reduction of alkyl isocyanides in presence of LiAlH4 yields secondary amines containing methyl as one of the alkyl group.

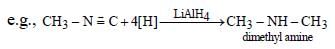
whereas, alkyl cyanides give 1° amine on reduction.
When aniline reacts with oil of bitter almonds(C6H5CHO) condensation takes place andbenzal derivative is formed. This is known as [1995]- a)Million's base
- b)Schiff's reagent
- c)Schiff's base
- d)Benedict's reagent
Correct answer is option 'C'. Can you explain this answer?
When aniline reacts with oil of bitter almonds(C6H5CHO) condensation takes place andbenzal derivative is formed. This is known as [1995]
a)
Million's base
b)
Schiff's reagent
c)
Schiff's base
d)
Benedict's reagent
|
|
Geetika Tiwari answered |
Aniline is an organic compound with the chemical formula C6H5NH2. Oil of bitter almonds, also known as benzaldehyde, has the chemical formula C6H5CHO. When aniline reacts with oil of bitter almonds, a condensation reaction takes place, resulting in the formation of a benzal derivative.
The reaction between aniline and oil of bitter almonds is known as the Schiff's base reaction. Schiff's base is a class of organic compounds that are formed by the condensation of a primary amine with an aldehyde or ketone. In this case, aniline acts as the primary amine, and oil of bitter almonds acts as the aldehyde.
The reaction mechanism involves the nucleophilic attack of the amino group of aniline on the carbonyl carbon of the aldehyde group in the oil of bitter almonds. This leads to the formation of a carbon-nitrogen double bond, known as an imine linkage. The imine formed is the benzal derivative.
The Schiff's base reaction is an important reaction in organic chemistry because it allows for the synthesis of a wide range of compounds, including dyes, pharmaceuticals, and natural products. It is named after the German chemist Hugo Schiff, who first described this type of reaction in the late 19th century.
In the given options, the correct answer is option C - Schiff's base. The reaction between aniline and oil of bitter almonds is a classic example of a Schiff's base reaction. The formation of the benzal derivative through condensation is a characteristic feature of this reaction.
Overall, the reaction between aniline and oil of bitter almonds is an example of a Schiff's base reaction, where a condensation reaction takes place, resulting in the formation of a benzal derivative. This reaction has important applications in organic synthesis and is widely used in the preparation of various compounds.
The reaction between aniline and oil of bitter almonds is known as the Schiff's base reaction. Schiff's base is a class of organic compounds that are formed by the condensation of a primary amine with an aldehyde or ketone. In this case, aniline acts as the primary amine, and oil of bitter almonds acts as the aldehyde.
The reaction mechanism involves the nucleophilic attack of the amino group of aniline on the carbonyl carbon of the aldehyde group in the oil of bitter almonds. This leads to the formation of a carbon-nitrogen double bond, known as an imine linkage. The imine formed is the benzal derivative.
The Schiff's base reaction is an important reaction in organic chemistry because it allows for the synthesis of a wide range of compounds, including dyes, pharmaceuticals, and natural products. It is named after the German chemist Hugo Schiff, who first described this type of reaction in the late 19th century.
In the given options, the correct answer is option C - Schiff's base. The reaction between aniline and oil of bitter almonds is a classic example of a Schiff's base reaction. The formation of the benzal derivative through condensation is a characteristic feature of this reaction.
Overall, the reaction between aniline and oil of bitter almonds is an example of a Schiff's base reaction, where a condensation reaction takes place, resulting in the formation of a benzal derivative. This reaction has important applications in organic synthesis and is widely used in the preparation of various compounds.
On hydrolysis of a “compound”, twocompounds are obtained. One of which ontreatment with sodium nitrite and hydrochloricacid gives a product which does not respond toiodoform test. The second one reduces Tollen’sreagent and Fehling’s solution. The “compound”is [NEET Kar. 2013]- a)CH3 CH2 CH2 CON(CH3)2
- b)CH3 CH2 CH2 NC
- c)CH3 CH2 CH2 CN
- d)CH3 CH2 CH2 ON = O
Correct answer is option 'B'. Can you explain this answer?
On hydrolysis of a “compound”, twocompounds are obtained. One of which ontreatment with sodium nitrite and hydrochloricacid gives a product which does not respond toiodoform test. The second one reduces Tollen’sreagent and Fehling’s solution. The “compound”is [NEET Kar. 2013]
a)
CH3 CH2 CH2 CON(CH3)2
b)
CH3 CH2 CH2 NC
c)
CH3 CH2 CH2 CN
d)
CH3 CH2 CH2 ON = O

|
Dipika Das answered |
Hydrolysis gives CH3CH2CH2NH2 +
HCOOH. On treatment with NaNO2 and
HCl former gives CH3CH2CH2OH which
does not give iodoform test. HCOOH gives Tollen’s reagent test.
HCOOH. On treatment with NaNO2 and
HCl former gives CH3CH2CH2OH which
does not give iodoform test. HCOOH gives Tollen’s reagent test.
Mark the correct statement [1985]- a)Methylamine is slightly acidic
- b)Methylamine is less basic than ammonia
- c)Methylamine is a stronger base thanammonia
- d)Methylamine forms salts with alkalies.
Correct answer is option 'C'. Can you explain this answer?
Mark the correct statement [1985]
a)
Methylamine is slightly acidic
b)
Methylamine is less basic than ammonia
c)
Methylamine is a stronger base thanammonia
d)
Methylamine forms salts with alkalies.

|
Anand Jain answered |
Methyl amine is a stronger base than
ammonia due to +I effect. The alkyl groups
which are electron releasing groups increase
the electron density around the nitrogen
thereby increasing the availability of the lone
pair of electrons to proton or lewis acid and
making the amine more basic
ammonia due to +I effect. The alkyl groups
which are electron releasing groups increase
the electron density around the nitrogen
thereby increasing the availability of the lone
pair of electrons to proton or lewis acid and
making the amine more basic
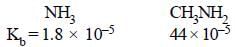
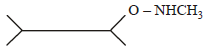
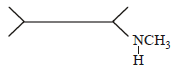
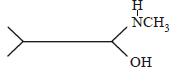
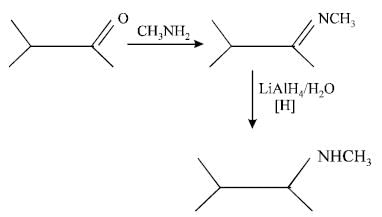
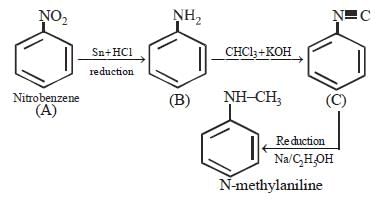
An organic compound (C3H9N) (A), when treated with nitrous acid, gave an alcohol and N2 gas was evolved. (A) on warming with CHCl3 and caustic potash gave (C) which on reduction gave isopropylmethylamine. Predict the structure of (A). [2012 M]- a)
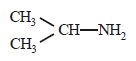
- b)CH3CH2 — NH — CH3
- c)
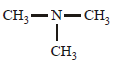
- d)CH3CH2CH2 — NH2
Correct answer is option 'A'. Can you explain this answer?
An organic compound (C3H9N) (A), when treated with nitrous acid, gave an alcohol and N2 gas was evolved. (A) on warming with CHCl3 and caustic potash gave (C) which on reduction gave isopropylmethylamine. Predict the structure of (A). [2012 M]
a)
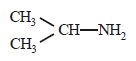
b)
CH3CH2 — NH — CH3
c)
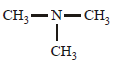
d)
CH3CH2CH2 — NH2

|
Smruti Sucharita answered |
This is the carbyl amine reaction....
Primary amines gives this test..... Here in 2 options primary amines are there....
But on reduction the product is isopropyl methylamine.... So the last option is correct...
Primary amines gives this test..... Here in 2 options primary amines are there....
But on reduction the product is isopropyl methylamine.... So the last option is correct...
Which is formed, when acetonitrile is hydrolysedpartially with cold concentrated HCl? [1995]- a)acetic acid
- b)acetamide
- c)methyl cyanide
- d)acetic anhydrides
Correct answer is option 'B'. Can you explain this answer?
Which is formed, when acetonitrile is hydrolysedpartially with cold concentrated HCl? [1995]
a)
acetic acid
b)
acetamide
c)
methyl cyanide
d)
acetic anhydrides

|
Shruti Chauhan answered |
Methyl cyanide on treatment with conc. HCl give acetamide.
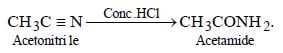
The compound obtained by heating a mixture ofa primary amine and chloroform with ethanolicpotassium hydroxide (KOH) is [1997]- a)an alkyl cyanide
- b)a nitro compound
- c)an alkyl isocyanide
- d)an amide
Correct answer is option 'C'. Can you explain this answer?
The compound obtained by heating a mixture ofa primary amine and chloroform with ethanolicpotassium hydroxide (KOH) is [1997]
a)
an alkyl cyanide
b)
a nitro compound
c)
an alkyl isocyanide
d)
an amide

|
Vaibhav Basu answered |
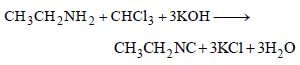
In this reaction, bad smelling compound ethyl isocyanide (CH3CH2NC) is produced. This equation is known as carbyl amine reaction.
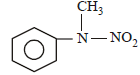
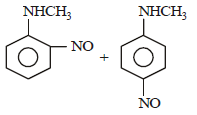
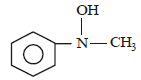
Predict the product: [2009]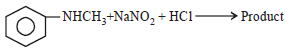
- a)
- b)
- c)
- d)
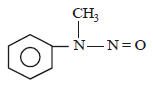
Correct answer is option 'D'. Can you explain this answer?
Predict the product: [2009]
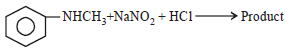
a)

b)

c)

d)


|
Nayanika Reddy answered |
Secondary amine with (NaNO2 + HCl) gives nitroso product
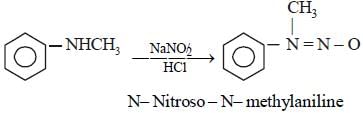
Which of the following compounds is most basic? [2011 M]- a)
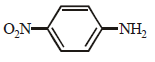
- b)
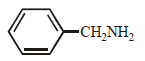
- c)
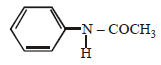
- d)
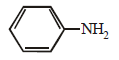
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds is most basic? [2011 M]
a)

b)

c)
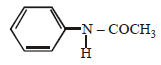
d)
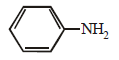

|
Arya Khanna answered |
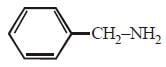 compound is most basic due to localized lone pair of electron on nitrogen atom while other compounds have delocalized lone pair of electron.
compound is most basic due to localized lone pair of electron on nitrogen atom while other compounds have delocalized lone pair of electron.Which of the following is most basic in nature? [2000]- a)NH3
- b)CH3NH2
- c)(CH3)2NH
- d)C6H5NHCH3
Correct answer is option 'C'. Can you explain this answer?
Which of the following is most basic in nature? [2000]
a)
NH3
b)
CH3NH2
c)
(CH3)2NH
d)
C6H5NHCH3

|
Arya Nair answered |
(CH3)2NH is most basic because two electron releasing groups are present on Nitrogen. Also aromatic amines are less basic then aliphatic amines. The basic character of amines follow
the order
R2NH > RNH2 > C6H5NHCH3 > NH3
the order
R2NH > RNH2 > C6H5NHCH3 > NH3
Which of the following is more basic thananiline? [2006]- a)Triphenylamine
- b)p-Nitroaniline
- c)Benzylamine
- d)Diphenylamine
Correct answer is option 'C'. Can you explain this answer?
Which of the following is more basic thananiline? [2006]
a)
Triphenylamine
b)
p-Nitroaniline
c)
Benzylamine
d)
Diphenylamine

|
Vaibhav Basu answered |
Benzylamine is more basic than aniline. The reason is that in aniline, the lone pair of nitrogen is conjugated with benzene ring so it is not available readily for others. On the other hand in Benzylamine, nitrogen is not directly attached with ring so lone pairs are
not conjugated with ring.
not conjugated with ring.
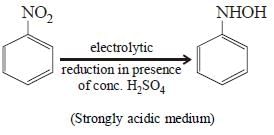
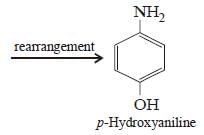
Electrolytic reduction of nitrobenzene in weaklyacidic medium gives [2005]- a)N-Phenylhydroxylamine
- b)Nitrosobenzene
- c)Aniline
- d)p-Hydroxyaniline
Correct answer is option 'C'. Can you explain this answer?
Electrolytic reduction of nitrobenzene in weaklyacidic medium gives [2005]
a)
N-Phenylhydroxylamine
b)
Nitrosobenzene
c)
Aniline
d)
p-Hydroxyaniline

|
Shalini Saha answered |
Electrolytic reduction of Nitroalkane in
weakly acidic medium give aniline
weakly acidic medium give aniline

Whereas in strongly acidic medium it gives p-hydroxyaniline
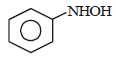
What is the product obtained in the following reaction : [2011]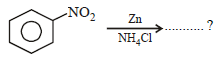
- a)
- b)
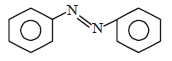
- c)
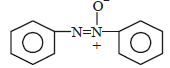
- d)
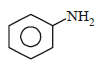
Correct answer is option 'A'. Can you explain this answer?
What is the product obtained in the following reaction : [2011]
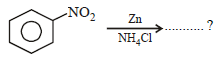
a)

b)
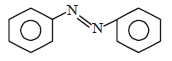
c)
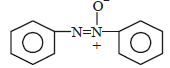
d)


|
Shivani Rane answered |
When nitro compound is reduced with a
neutral reducing agent (Zn dust + NH4Cl) the corresponding hydroxyl amine is formed
neutral reducing agent (Zn dust + NH4Cl) the corresponding hydroxyl amine is formed
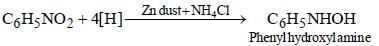
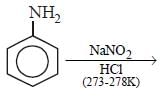
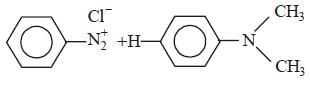
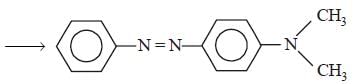
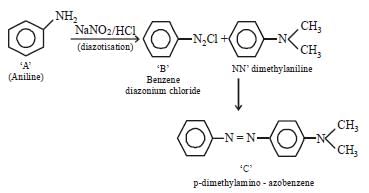
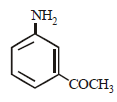
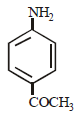
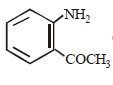
In a reaction of aniline a coloured product C was obtained. [2008]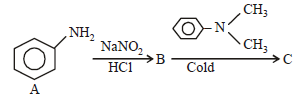 The structure of C would be :
The structure of C would be :- a)
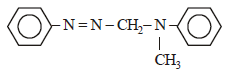
- b)
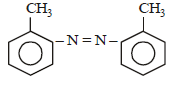
- c)
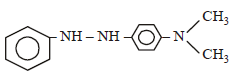
- d)
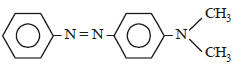
Correct answer is option 'D'. Can you explain this answer?
In a reaction of aniline a coloured product C was obtained. [2008]

The structure of C would be :
a)
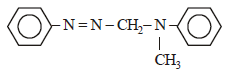
b)
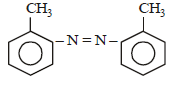
c)
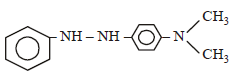
d)
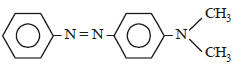

|
Ayush Sengupta answered |
The reaction can be completed as follows:
Aniline is an activated system for electrophilic substitution. The compound formed on heating aniline with acetic anhydride is [1996]- a)
- b)
- c)
- d)
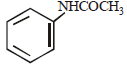
Correct answer is option 'D'. Can you explain this answer?
Aniline is an activated system for electrophilic substitution. The compound formed on heating aniline with acetic anhydride is [1996]
a)

b)

c)

d)


|
Krish Patel answered |
Aniline when treated with acetic anhydride forms acetanilide (nucleophilic substitution)
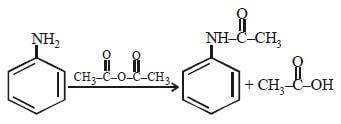
Some reactions of amines are given. Which one is not correct ? [NEET Kar. 2013]- a)
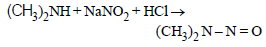
- b)
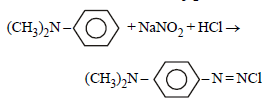
- c)
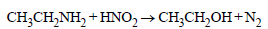
- d)
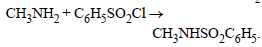
Correct answer is option 'B'. Can you explain this answer?
Some reactions of amines are given. Which one is not correct ? [NEET Kar. 2013]
a)
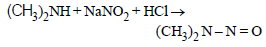
b)
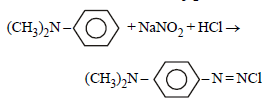
c)
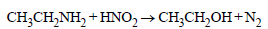
d)
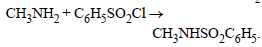

|
Sneha Basak answered |
Secondary amine react with nitrous acid to give N-Nitrosoamines.
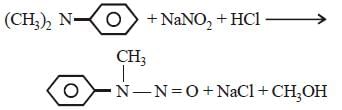
Which of the following statements about primaryamines is ‘False’ ? [2010]- a)Alkyl amines are stronger bases than arylamines
- b)Alkyl amines react with nitrous acid toproduce alcohols
- c)Aryl amines react with nitrous acid toproduce phenols
- d)Alkyl amines are stronger bases thanammonia
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements about primaryamines is ‘False’ ? [2010]
a)
Alkyl amines are stronger bases than arylamines
b)
Alkyl amines react with nitrous acid toproduce alcohols
c)
Aryl amines react with nitrous acid toproduce phenols
d)
Alkyl amines are stronger bases thanammonia

|
Shalini Saha answered |
Aryl amines do not produce phenol on
treatment with nitrous acid
treatment with nitrous acid
Match the compounds given in List - I with their characteristic reactions given in List - II. Select the correct option. [2010]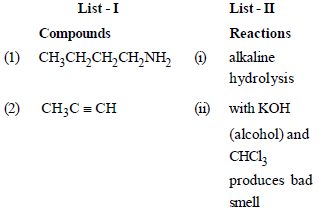
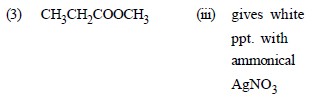
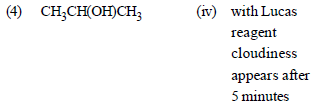 options :
options :
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
Match the compounds given in List - I with their characteristic reactions given in List - II. Select the correct option. [2010]

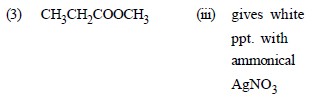
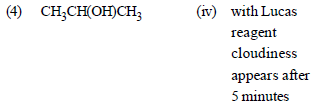
options :
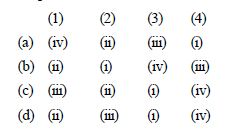
a)
a
b)
b
c)
c
d)
d

|
Soumya Ahuja answered |
(1) CH3 CH2 – CH2 – CH2 – NH2
(ii) with KOH (alcohol) and CHCl3
produces bad smell
(2) CH3C ≡ CH
(ii) gives white ppt with ammonical
AgNO3
(3) CH3 CH2 COOCH3
(i) alkaline hydrolysis
(4) CH3 CHOH – CH3
(iv) with Lucas reagent cloudiness
appears after 5 minutes
(ii) with KOH (alcohol) and CHCl3
produces bad smell
(2) CH3C ≡ CH
(ii) gives white ppt with ammonical
AgNO3
(3) CH3 CH2 COOCH3
(i) alkaline hydrolysis
(4) CH3 CHOH – CH3
(iv) with Lucas reagent cloudiness
appears after 5 minutes
Indicate which nitrogen compound amongst thefollowing would undergo Hofmann reaction (i.e..,reaction with Br2 and strong KOH) to furnish theprimary amine (R – NH2) [1989]- a)RCONHCH3
- b)RCOONH4
- c)RCONH2
- d)R —CO— NHOH.
Correct answer is option 'C'. Can you explain this answer?
Indicate which nitrogen compound amongst thefollowing would undergo Hofmann reaction (i.e..,reaction with Br2 and strong KOH) to furnish theprimary amine (R – NH2) [1989]
a)
RCONHCH3
b)
RCOONH4
c)
RCONH2
d)
R —CO— NHOH.
|
|
Suhana Agrawal answered |
Hoffmann reaction is the method of preparation of primary amines by treating an ' AMIDE ' with bromine in an aqueous or ethanol solution of sodium hydroxide.
hence option C being an amide is the correct answer.
hence option C being an amide is the correct answer.
Chapter doubts & questions for Amines - NEET Past Year Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Amines - NEET Past Year Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup