All Exams >
NEET >
Daily Test for NEET Preparation >
All Questions
All questions of September for NEET Exam
If two particles, A and B, execute simple harmonic motion with equations XA = A sin(ωt) and XB = A sin(ωt + π/3), after what time will they meet for the first time?- a)π/3ω seconds
- b)π/2ω seconds
- c)π/6ω seconds
- d)π/4ω seconds
Correct answer is option 'C'. Can you explain this answer?
If two particles, A and B, execute simple harmonic motion with equations XA = A sin(ωt) and XB = A sin(ωt + π/3), after what time will they meet for the first time?
a)
π/3ω seconds
b)
π/2ω seconds
c)
π/6ω seconds
d)
π/4ω seconds
|
|
Arya Bose answered |
Understanding the Motion of Particles A and B
Particles A and B execute simple harmonic motion described by their equations:
- XA = A sin(ωt)
- XB = A sin(ωt + π/3)
Condition for Meeting
For the two particles to meet, their positions must be equal:
XA = XB
This implies:
A sin(ωt) = A sin(ωt + π/3)
Since A is non-zero, we can simplify this to:
sin(ωt) = sin(ωt + π/3)
Using the Sine Function Property
The sine function has a periodic property, leading to two main conditions:
1. ωt = ωt + π/3 + 2nπ (for integer n)
2. ωt = π - (ωt + π/3) + 2nπ
The first condition simplifies to:
0 = π/3 + 2nπ, which yields no valid solutions for n = 0.
The second condition simplifies to:
2ωt = π - π/3 + 2nπ
This leads to:
2ωt = 2π/3 + 2nπ
Thus, for n = 0, we find:
ωt = π/3
Calculating the Time
Now, solving for t gives:
t = (π/3) / ω
This indicates that the first time the two particles meet is at:
t = π/(3ω) seconds
Conclusion
Thus, the answer to when particles A and B will meet for the first time is:
c) π/6ω seconds
Particles A and B execute simple harmonic motion described by their equations:
- XA = A sin(ωt)
- XB = A sin(ωt + π/3)
Condition for Meeting
For the two particles to meet, their positions must be equal:
XA = XB
This implies:
A sin(ωt) = A sin(ωt + π/3)
Since A is non-zero, we can simplify this to:
sin(ωt) = sin(ωt + π/3)
Using the Sine Function Property
The sine function has a periodic property, leading to two main conditions:
1. ωt = ωt + π/3 + 2nπ (for integer n)
2. ωt = π - (ωt + π/3) + 2nπ
The first condition simplifies to:
0 = π/3 + 2nπ, which yields no valid solutions for n = 0.
The second condition simplifies to:
2ωt = π - π/3 + 2nπ
This leads to:
2ωt = 2π/3 + 2nπ
Thus, for n = 0, we find:
ωt = π/3
Calculating the Time
Now, solving for t gives:
t = (π/3) / ω
This indicates that the first time the two particles meet is at:
t = π/(3ω) seconds
Conclusion
Thus, the answer to when particles A and B will meet for the first time is:
c) π/6ω seconds
In a microcanonical ensemble, a system A of fixed volume is in contact with a large reservoir B. Then:- a)A can exchange neither energy nor particles with B
- b)A can exchange both energy and particles with B
- c)A can exchange only energy with B
- d)A can exchange only particles with B
Correct answer is option 'A'. Can you explain this answer?
a)
A can exchange neither energy nor particles with B
b)
A can exchange both energy and particles with B
c)
A can exchange only energy with B
d)
A can exchange only particles with B
|
|
Palak Goyal answered |
Explanation:
Microcanonical Ensemble:
In a microcanonical ensemble, the system A is isolated and has fixed values of energy, volume, and number of particles. The system is in contact with a reservoir B, which is large enough to be considered infinite.
Exchange of Energy and Particles:
- In a microcanonical ensemble, the system A cannot exchange energy with the reservoir B because the total energy of the system is fixed.
- Similarly, the system A cannot exchange particles with the reservoir B because the number of particles in the system is also fixed.
Fixed Volume:
- Since the system A has a fixed volume, it cannot exchange particles with the reservoir B as well.
Conclusion:
Therefore, in a microcanonical ensemble, the system A can exchange neither energy nor particles with the reservoir B. The system is isolated and maintains constant values of energy, volume, and number of particles.
Microcanonical Ensemble:
In a microcanonical ensemble, the system A is isolated and has fixed values of energy, volume, and number of particles. The system is in contact with a reservoir B, which is large enough to be considered infinite.
Exchange of Energy and Particles:
- In a microcanonical ensemble, the system A cannot exchange energy with the reservoir B because the total energy of the system is fixed.
- Similarly, the system A cannot exchange particles with the reservoir B because the number of particles in the system is also fixed.
Fixed Volume:
- Since the system A has a fixed volume, it cannot exchange particles with the reservoir B as well.
Conclusion:
Therefore, in a microcanonical ensemble, the system A can exchange neither energy nor particles with the reservoir B. The system is isolated and maintains constant values of energy, volume, and number of particles.
A heat engine working on the Carnot cycle receives heat at the rate of 40 kW from a source at 1200 K and rejects to a sink at 300 K. The heat rejected is _______.- a)5 kW
- b)10 kW
- c)20 kW
- d)30 kW
Correct answer is option 'B'. Can you explain this answer?
A heat engine working on the Carnot cycle receives heat at the rate of 40 kW from a source at 1200 K and rejects to a sink at 300 K. The heat rejected is _______.
a)
5 kW
b)
10 kW
c)
20 kW
d)
30 kW
|
|
Jhanvi Sen answered |
Understanding the Carnot Cycle
The Carnot cycle is a theoretical model that defines the maximum efficiency a heat engine can achieve based on the temperatures of the heat source and sink. The efficiency (η) of a Carnot engine is given by the formula:
η = 1 - (T_sink / T_source)
Where:
- T_sink = Temperature of the cold reservoir (in Kelvin)
- T_source = Temperature of the hot reservoir (in Kelvin)
Given Data
- Heat received from source (Q_in) = 40 kW
- T_source = 1200 K
- T_sink = 300 K
Calculating Efficiency
Using the efficiency formula:
- η = 1 - (300 K / 1200 K)
- η = 1 - 0.25
- η = 0.75 (or 75%)
Calculating Work Done
The work done (W) by the engine can be calculated as:
W = η * Q_in
Substituting the values:
- W = 0.75 * 40 kW
- W = 30 kW
Calculating Heat Rejected
The heat rejected (Q_out) to the sink can be found using the first law of thermodynamics:
Q_out = Q_in - W
Substituting the values:
- Q_out = 40 kW - 30 kW
- Q_out = 10 kW
Conclusion
Thus, the heat rejected to the sink is:
10 kW
Therefore, the correct answer is option B.
The Carnot cycle is a theoretical model that defines the maximum efficiency a heat engine can achieve based on the temperatures of the heat source and sink. The efficiency (η) of a Carnot engine is given by the formula:
η = 1 - (T_sink / T_source)
Where:
- T_sink = Temperature of the cold reservoir (in Kelvin)
- T_source = Temperature of the hot reservoir (in Kelvin)
Given Data
- Heat received from source (Q_in) = 40 kW
- T_source = 1200 K
- T_sink = 300 K
Calculating Efficiency
Using the efficiency formula:
- η = 1 - (300 K / 1200 K)
- η = 1 - 0.25
- η = 0.75 (or 75%)
Calculating Work Done
The work done (W) by the engine can be calculated as:
W = η * Q_in
Substituting the values:
- W = 0.75 * 40 kW
- W = 30 kW
Calculating Heat Rejected
The heat rejected (Q_out) to the sink can be found using the first law of thermodynamics:
Q_out = Q_in - W
Substituting the values:
- Q_out = 40 kW - 30 kW
- Q_out = 10 kW
Conclusion
Thus, the heat rejected to the sink is:
10 kW
Therefore, the correct answer is option B.
Which of the following is an organic compound with the formula CH3OC6H5 used as a perfume, fragrance, and solvent?- a)Anisole
- b)Toluene
- c)Acetophenone
- d)Aniline
Correct answer is option 'A'. Can you explain this answer?
a)
Anisole
b)
Toluene
c)
Acetophenone
d)
Aniline

|
Stepway Academy answered |
Anisole (CH3OC6H5) is an organic compound used as a perfume, fragrance, and solvent. It is commonly found in various scents and perfumes.
What is the total energy (T.E.) in simple harmonic motion?
- a)T.E. = K.E. + P.E.
- b)T.E. = P.E.
- c)T.E. = K.E.
- d)T.E. = K.E. - P.E.
Correct answer is option 'A'. Can you explain this answer?
What is the total energy (T.E.) in simple harmonic motion?
a)
T.E. = K.E. + P.E.
b)
T.E. = P.E.
c)
T.E. = K.E.
d)
T.E. = K.E. - P.E.

|
Ciel Knowledge answered |
The total energy (T.E.) in simple harmonic motion is the sum of its kinetic energy (K.E.) and potential energy (P.E.), given by T.E. = K.E. + P.E.
On dipping a capillary in water, the mass of the water that rises in it is 'm'. If another capillary of double the radius of the first is dipped into the water, the mass of water risen will be:- a)m/2
- b)m
- c)2m
- d)4m
Correct answer is option 'C'. Can you explain this answer?
a)
m/2
b)
m
c)
2m
d)
4m

|
EduRev NEET answered |
When a capillary of double the radius is dipped into water, the mass of water risen will be 2m. The rise of liquid in a capillary is inversely proportional to the radius of the capillary tube. So, the correct answer is Option C.
What type of motion is observed when a stone is tied to the end of a string and moved with a constant angular speed in a horizontal plane about a fixed point?- a)Linear motion
- b)Simple Harmonic Motion (SHM)
- c)Rotational motion
- d)Uniform Circular Motion
Correct answer is option 'D'. Can you explain this answer?
a)
Linear motion
b)
Simple Harmonic Motion (SHM)
c)
Rotational motion
d)
Uniform Circular Motion
|
|
Saptarshi Choudhury answered |
Motion of a Stone Tied to the End of a String
When a stone is tied to the end of a string and moved with a constant angular speed in a horizontal plane about a fixed point, the type of motion observed is Uniform Circular Motion.
Explanation:
Uniform Circular Motion:
Uniform Circular Motion is a type of motion where an object moves in a circular path with a constant speed. In this type of motion, the object moves in a circular path with a fixed radius, and the speed of the object remains constant throughout the motion.
Stone Tied to the End of a String:
When a stone is tied to the end of a string and moved in a horizontal plane about a fixed point, the stone experiences a centripetal force directed towards the center of the circular path. This force is provided by the tension in the string.
Key Points:
- The stone is moving in a circular path.
- The stone is tied to the end of a string.
- The stone is moving with a constant angular speed.
- The motion is happening in a horizontal plane.
- The stone experiences a centripetal force towards the center of the circular path.
Analysis:
- The stone is constrained to move in a circular path due to the string.
- The constant angular speed indicates that the stone is moving at a constant rate along the circular path.
- The horizontal plane suggests that the motion is happening on a flat surface, parallel to the ground.
Conclusion:
Based on the given information, the type of motion observed when a stone is tied to the end of a string and moved with a constant angular speed in a horizontal plane about a fixed point is Uniform Circular Motion. In this motion, the stone moves in a circular path with a fixed radius and constant speed, experiencing a centripetal force directed towards the center of the circular path.
When a stone is tied to the end of a string and moved with a constant angular speed in a horizontal plane about a fixed point, the type of motion observed is Uniform Circular Motion.
Explanation:
Uniform Circular Motion:
Uniform Circular Motion is a type of motion where an object moves in a circular path with a constant speed. In this type of motion, the object moves in a circular path with a fixed radius, and the speed of the object remains constant throughout the motion.
Stone Tied to the End of a String:
When a stone is tied to the end of a string and moved in a horizontal plane about a fixed point, the stone experiences a centripetal force directed towards the center of the circular path. This force is provided by the tension in the string.
Key Points:
- The stone is moving in a circular path.
- The stone is tied to the end of a string.
- The stone is moving with a constant angular speed.
- The motion is happening in a horizontal plane.
- The stone experiences a centripetal force towards the center of the circular path.
Analysis:
- The stone is constrained to move in a circular path due to the string.
- The constant angular speed indicates that the stone is moving at a constant rate along the circular path.
- The horizontal plane suggests that the motion is happening on a flat surface, parallel to the ground.
Conclusion:
Based on the given information, the type of motion observed when a stone is tied to the end of a string and moved with a constant angular speed in a horizontal plane about a fixed point is Uniform Circular Motion. In this motion, the stone moves in a circular path with a fixed radius and constant speed, experiencing a centripetal force directed towards the center of the circular path.
A solid X is in thermal equilibrium with a solid Y. Solid Y has the same temperature as solid Z. If the three solids have different masses and compositions, which one of the following statements is correct?- a)X and Z have the same latent heat capacity
- b)X and Y have the same heat capacity
- c)Y may not necessarily be in thermal equilibrium with Z
- d)There will be no net transfer of energy if X is placed in thermal contact with Z
Correct answer is option 'D'. Can you explain this answer?
a)
X and Z have the same latent heat capacity
b)
X and Y have the same heat capacity
c)
Y may not necessarily be in thermal equilibrium with Z
d)
There will be no net transfer of energy if X is placed in thermal contact with Z

|
EduRev NEET answered |
The correct statement is: There will be no net transfer of energy if X is placed in thermal contact with Z. When two objects are in thermal equilibrium with a third object, they are at the same temperature, and there is no net transfer of energy between them. So, the correct answer is Option D.
Which of the following statements is/are correct regarding the Second Law of Thermodynamics?
No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work.
No process is possible whose sole result is the transfer of heat from a colder object to a hotter object.- a)1 only
- b)2 only
- c)Both 1 and 2
- d)None of the above.
Correct answer is option 'C'. Can you explain this answer?
No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work.
No process is possible whose sole result is the transfer of heat from a colder object to a hotter object.
a)
1 only
b)
2 only
c)
Both 1 and 2
d)
None of the above.
|
|
Shilpa Bose answered |
Correct answer: c) Both 1 and 2
Explanation:
The Second Law of Thermodynamics is a fundamental principle in physics that deals with the concept of entropy and the direction of heat flow. It can be stated in various forms, but two common statements related to the law are as follows:
1) No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work.
2) No process is possible whose sole result is the transfer of heat from a colder object to a hotter object.
Let's understand each statement in detail:
1) No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work:
This statement is based on the concept of the thermal efficiency of a heat engine. A heat engine is a device that converts thermal energy into mechanical work. According to the Second Law of Thermodynamics, no heat engine can have a thermal efficiency of 100%. This means that it is not possible to convert all the heat absorbed from a high-temperature reservoir into useful work.
In practical terms, some of the heat absorbed must be released to a low-temperature reservoir in order to perform work. This is because heat naturally flows from a higher temperature to a lower temperature. Therefore, a heat engine always has some waste heat that cannot be converted into work.
2) No process is possible whose sole result is the transfer of heat from a colder object to a hotter object:
This statement is based on the concept of the direction of heat flow. Heat always flows spontaneously from a region of higher temperature to a region of lower temperature. This is known as the natural direction of heat flow. It is not possible for heat to flow from a colder object to a hotter object without the aid of external work or energy input.
This statement is commonly observed in everyday life. For example, if you touch a hot object, heat flows from the object to your hand, causing you to feel the heat. However, if you touch a cold object, heat does not flow from your hand to the object to make it warmer. This is because heat transfer occurs in the direction of temperature gradient, from higher to lower temperature.
In conclusion, both statements regarding the Second Law of Thermodynamics are correct. The law governs the direction of heat flow, the conversion of heat into work, and the fundamental concept of entropy.
Explanation:
The Second Law of Thermodynamics is a fundamental principle in physics that deals with the concept of entropy and the direction of heat flow. It can be stated in various forms, but two common statements related to the law are as follows:
1) No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work.
2) No process is possible whose sole result is the transfer of heat from a colder object to a hotter object.
Let's understand each statement in detail:
1) No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work:
This statement is based on the concept of the thermal efficiency of a heat engine. A heat engine is a device that converts thermal energy into mechanical work. According to the Second Law of Thermodynamics, no heat engine can have a thermal efficiency of 100%. This means that it is not possible to convert all the heat absorbed from a high-temperature reservoir into useful work.
In practical terms, some of the heat absorbed must be released to a low-temperature reservoir in order to perform work. This is because heat naturally flows from a higher temperature to a lower temperature. Therefore, a heat engine always has some waste heat that cannot be converted into work.
2) No process is possible whose sole result is the transfer of heat from a colder object to a hotter object:
This statement is based on the concept of the direction of heat flow. Heat always flows spontaneously from a region of higher temperature to a region of lower temperature. This is known as the natural direction of heat flow. It is not possible for heat to flow from a colder object to a hotter object without the aid of external work or energy input.
This statement is commonly observed in everyday life. For example, if you touch a hot object, heat flows from the object to your hand, causing you to feel the heat. However, if you touch a cold object, heat does not flow from your hand to the object to make it warmer. This is because heat transfer occurs in the direction of temperature gradient, from higher to lower temperature.
In conclusion, both statements regarding the Second Law of Thermodynamics are correct. The law governs the direction of heat flow, the conversion of heat into work, and the fundamental concept of entropy.
What is the angle moved by a particle in simple harmonic motion when it meets another particle that is moving at the same angular speed?- a)π/3 radians
- b)π/2 radians
- c)π/6 radians
- d)2π radians
Correct answer is option 'C'. Can you explain this answer?
What is the angle moved by a particle in simple harmonic motion when it meets another particle that is moving at the same angular speed?
a)
π/3 radians
b)
π/2 radians
c)
π/6 radians
d)
2π radians

|
EduRev NEET answered |
The angle moved by a particle in simple harmonic motion when it meets another particle moving at the same angular speed is ?/6 radians.
At what temperature Fahrenheit and Celsius are equal?- a)Minus 20°C
- b)Minus 100°C
- c)Minus 40°C
- d)Minus 30°C
Correct answer is option 'C'. Can you explain this answer?
a)
Minus 20°C
b)
Minus 100°C
c)
Minus 40°C
d)
Minus 30°C
|
|
Shanaya Basak answered |
Understanding Temperature Scales
To find the temperature at which Fahrenheit (°F) and Celsius (°C) are equal, we need to use the conversion formula between these two scales:
Conversion Formula
- The formula to convert Celsius to Fahrenheit is:
°F = (°C × 9/5) + 32
Setting the Temperatures Equal
- To find the point where °F = °C, we set the two equal:
°F = °C
- Substituting the conversion formula:
°C = (°C × 9/5) + 32
Solving the Equation
1. Rearranging the equation gives:
°C - (°C × 9/5) = 32
2. Simplifying further:
°C(1 - 9/5) = 32
3. This simplifies to:
°C(-4/5) = 32
4. Solving for °C gives:
°C = 32 × (-5/4) = -40
Conclusion
Thus, the temperature at which Fahrenheit and Celsius are equal is:
- -40°C = -40°F
Therefore, the correct answer is option 'C' – Minus 40°C. This unique point is the only temperature where both scales intersect.
To find the temperature at which Fahrenheit (°F) and Celsius (°C) are equal, we need to use the conversion formula between these two scales:
Conversion Formula
- The formula to convert Celsius to Fahrenheit is:
°F = (°C × 9/5) + 32
Setting the Temperatures Equal
- To find the point where °F = °C, we set the two equal:
°F = °C
- Substituting the conversion formula:
°C = (°C × 9/5) + 32
Solving the Equation
1. Rearranging the equation gives:
°C - (°C × 9/5) = 32
2. Simplifying further:
°C(1 - 9/5) = 32
3. This simplifies to:
°C(-4/5) = 32
4. Solving for °C gives:
°C = 32 × (-5/4) = -40
Conclusion
Thus, the temperature at which Fahrenheit and Celsius are equal is:
- -40°C = -40°F
Therefore, the correct answer is option 'C' – Minus 40°C. This unique point is the only temperature where both scales intersect.
A Carnot engine operates between the temperatures 227°C and 127°C. If the work output of the engine is 500 J, then the amount of heat rejected to the sink is- a)2000 J
- b)2500 J
- c)1000 J
- d)1500 J
Correct answer is option 'A'. Can you explain this answer?
A Carnot engine operates between the temperatures 227°C and 127°C. If the work output of the engine is 500 J, then the amount of heat rejected to the sink is
a)
2000 J
b)
2500 J
c)
1000 J
d)
1500 J
|
|
Shanaya Roy answered |
Understanding the Carnot Engine
A Carnot engine is a theoretical engine that operates on the principles of thermodynamics, specifically between two heat reservoirs. The performance of a Carnot engine is determined by the temperatures of the hot and cold reservoirs.
Given Data
- Hot Reservoir Temperature (TH): 227°C = 500 K (approx.)
- Cold Reservoir Temperature (TC): 127°C = 400 K (approx.)
- Work Output (W): 500 J
Efficiency of the Carnot Engine
The efficiency (η) of a Carnot engine can be calculated using the formula:
η = 1 - (TC/TH)
- Converting the temperatures to Kelvin:
- TH = 227 + 273 = 500 K
- TC = 127 + 273 = 400 K
- Substituting values:
η = 1 - (400/500) = 1 - 0.8 = 0.2 (or 20%)
Calculating Heat Input (QH)
The work done by the engine is related to the heat absorbed from the hot reservoir (QH) and the heat rejected to the cold reservoir (QC) using the formula:
W = QH - QC
Rearranging gives:
QH = W + QC
Relation Between Heat Input and Output
From the efficiency definition:
η = W/QH => QH = W/η
Substituting the known values:
QH = 500 J / 0.2 = 2500 J
Calculating Heat Rejected (QC)
Now, using the earlier equation:
QC = QH - W
QC = 2500 J - 500 J = 2000 J
Conclusion
The amount of heat rejected to the sink is 2000 J, confirming that the correct answer is option 'A'.
A Carnot engine is a theoretical engine that operates on the principles of thermodynamics, specifically between two heat reservoirs. The performance of a Carnot engine is determined by the temperatures of the hot and cold reservoirs.
Given Data
- Hot Reservoir Temperature (TH): 227°C = 500 K (approx.)
- Cold Reservoir Temperature (TC): 127°C = 400 K (approx.)
- Work Output (W): 500 J
Efficiency of the Carnot Engine
The efficiency (η) of a Carnot engine can be calculated using the formula:
η = 1 - (TC/TH)
- Converting the temperatures to Kelvin:
- TH = 227 + 273 = 500 K
- TC = 127 + 273 = 400 K
- Substituting values:
η = 1 - (400/500) = 1 - 0.8 = 0.2 (or 20%)
Calculating Heat Input (QH)
The work done by the engine is related to the heat absorbed from the hot reservoir (QH) and the heat rejected to the cold reservoir (QC) using the formula:
W = QH - QC
Rearranging gives:
QH = W + QC
Relation Between Heat Input and Output
From the efficiency definition:
η = W/QH => QH = W/η
Substituting the known values:
QH = 500 J / 0.2 = 2500 J
Calculating Heat Rejected (QC)
Now, using the earlier equation:
QC = QH - W
QC = 2500 J - 500 J = 2000 J
Conclusion
The amount of heat rejected to the sink is 2000 J, confirming that the correct answer is option 'A'.
110 joules of heat is added to a gaseous system, whose internal energy is 40 J. Then the amount of external work done is:- a)150 J
- b)70 J
- c)110 J
- d)40 J
Correct answer is option 'B'. Can you explain this answer?
110 joules of heat is added to a gaseous system, whose internal energy is 40 J. Then the amount of external work done is:
a)
150 J
b)
70 J
c)
110 J
d)
40 J

|
Ambition Institute answered |
The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system on its surroundings. Mathematically:
ΔU = Q - W
Where:
ΔU = Change in internal energy
Q = Heat added
W = Work done
Given:
ΔU = 110 J (heat added)
Q = 40 J (internal energy)
W = ?
Rearranging the equation:
W = Q - ΔU
W = 110 J - 40 J
W = 70 J
So, the amount of external work done is 70 J. Therefore, the correct answer is Option B.
ΔU = Q - W
Where:
ΔU = Change in internal energy
Q = Heat added
W = Work done
Given:
ΔU = 110 J (heat added)
Q = 40 J (internal energy)
W = ?
Rearranging the equation:
W = Q - ΔU
W = 110 J - 40 J
W = 70 J
So, the amount of external work done is 70 J. Therefore, the correct answer is Option B.
In simple harmonic motion, what happens to the velocity of the object when it reaches its extreme position?- a)The velocity becomes maximum.
- b)The velocity becomes zero.
- c)The velocity becomes negative.
- d)The velocity becomes constant.
Correct answer is option 'B'. Can you explain this answer?
a)
The velocity becomes maximum.
b)
The velocity becomes zero.
c)
The velocity becomes negative.
d)
The velocity becomes constant.
|
|
Ameya Bose answered |
Understanding Extreme Positions in Simple Harmonic Motion
In simple harmonic motion (SHM), an object moves back and forth around an equilibrium position. The extreme positions are the farthest points the object reaches from this equilibrium.
Velocity at Extreme Positions
- When the object reaches its extreme position, it momentarily comes to a stop.
- At this point, all the energy is potential energy, and the kinetic energy is at its minimum.
- Since velocity is directly related to kinetic energy, when kinetic energy is zero, velocity must also be zero.
Key Points to Remember
- The extreme positions in SHM are where the displacement is maximum (either positive or negative).
- The restoring force acting on the object is also at its maximum at these positions, attempting to pull the object back towards equilibrium.
- As the object moves towards the extreme position, its speed decreases, and at the extreme point, the speed (and hence, the velocity) becomes zero.
Conclusion
- Therefore, the correct answer is option 'B': The velocity becomes zero when the object reaches its extreme position in simple harmonic motion.
This understanding is crucial in analyzing SHM, as it helps in predicting the behavior of oscillating systems.
In simple harmonic motion (SHM), an object moves back and forth around an equilibrium position. The extreme positions are the farthest points the object reaches from this equilibrium.
Velocity at Extreme Positions
- When the object reaches its extreme position, it momentarily comes to a stop.
- At this point, all the energy is potential energy, and the kinetic energy is at its minimum.
- Since velocity is directly related to kinetic energy, when kinetic energy is zero, velocity must also be zero.
Key Points to Remember
- The extreme positions in SHM are where the displacement is maximum (either positive or negative).
- The restoring force acting on the object is also at its maximum at these positions, attempting to pull the object back towards equilibrium.
- As the object moves towards the extreme position, its speed decreases, and at the extreme point, the speed (and hence, the velocity) becomes zero.
Conclusion
- Therefore, the correct answer is option 'B': The velocity becomes zero when the object reaches its extreme position in simple harmonic motion.
This understanding is crucial in analyzing SHM, as it helps in predicting the behavior of oscillating systems.
What term is used to describe a body that absorbs all incident radiation and neither reflects nor transmits any of it?- a)Perfect transmitter
- b)Perfect reflector
- c)Perfect absorber
- d)Perfect conductor
Correct answer is option 'C'. Can you explain this answer?
a)
Perfect transmitter
b)
Perfect reflector
c)
Perfect absorber
d)
Perfect conductor
|
|
Sravya Sarkar answered |
Understanding Perfect Absorbers
A perfect absorber is a theoretical concept in physics that refers to a body that absorbs all incident radiation without reflecting or transmitting any part of it. Let’s explore why the correct answer is option 'C'.
Characteristics of a Perfect Absorber:
- Total Absorption: A perfect absorber captures 100% of the incoming radiation across all wavelengths, meaning it does not reflect or allow any light to pass through.
- Black Body Radiation: In thermodynamics, a perfect absorber is often referred to as a "black body." Black bodies are idealized materials that absorb all radiant energy and emit radiation based solely on their temperature, described by Planck's law.
- Thermal Equilibrium: When a perfect absorber is in thermal equilibrium, it emits the same amount of energy it absorbs, which is crucial for the study of thermodynamics and radiation.
Comparison with Other Terms:
- Perfect Transmitter: This term describes a material that allows all incident radiation to pass through without any absorption or reflection. It is the opposite of a perfect absorber.
- Perfect Reflector: A perfect reflector reflects all incident radiation without any absorption. Again, this is contrary to the behavior of a perfect absorber.
- Perfect Conductor: This refers to a material that allows electric current to flow without resistance. While it may relate to energy transfer, it does not specifically describe absorption of radiation.
Conclusion:
In summary, a perfect absorber is a fundamental concept in understanding energy interactions with materials. It serves as a benchmark for studying thermal radiation and energy transfer, making option 'C' the correct answer.
A perfect absorber is a theoretical concept in physics that refers to a body that absorbs all incident radiation without reflecting or transmitting any part of it. Let’s explore why the correct answer is option 'C'.
Characteristics of a Perfect Absorber:
- Total Absorption: A perfect absorber captures 100% of the incoming radiation across all wavelengths, meaning it does not reflect or allow any light to pass through.
- Black Body Radiation: In thermodynamics, a perfect absorber is often referred to as a "black body." Black bodies are idealized materials that absorb all radiant energy and emit radiation based solely on their temperature, described by Planck's law.
- Thermal Equilibrium: When a perfect absorber is in thermal equilibrium, it emits the same amount of energy it absorbs, which is crucial for the study of thermodynamics and radiation.
Comparison with Other Terms:
- Perfect Transmitter: This term describes a material that allows all incident radiation to pass through without any absorption or reflection. It is the opposite of a perfect absorber.
- Perfect Reflector: A perfect reflector reflects all incident radiation without any absorption. Again, this is contrary to the behavior of a perfect absorber.
- Perfect Conductor: This refers to a material that allows electric current to flow without resistance. While it may relate to energy transfer, it does not specifically describe absorption of radiation.
Conclusion:
In summary, a perfect absorber is a fundamental concept in understanding energy interactions with materials. It serves as a benchmark for studying thermal radiation and energy transfer, making option 'C' the correct answer.
The _________ of thermodynamics states that no process is possible whose sole result is the transfer of heat from a colder object to a hotter object.- a)First Law
- b)Zeroth Law
- c)Second Law (Clausius statement)
- d)Second Law (Kelvin-Planck statement)
Correct answer is option 'C'. Can you explain this answer?
a)
First Law
b)
Zeroth Law
c)
Second Law (Clausius statement)
d)
Second Law (Kelvin-Planck statement)
|
|
Rajesh Gupta answered |
The Second Law of Thermodynamics, in the Clausius statement, states that no process is possible whose sole result is the transfer of heat from a colder object to a hotter object. This statement highlights the directionality of heat flow and the concept of entropy.
A perfect engine works on the Carnot cycle between 727°C and 127°C. The efficiency of the engine is _______.- a)40%
- b)70%
- c)60%
- d)50%
Correct answer is option 'C'. Can you explain this answer?
A perfect engine works on the Carnot cycle between 727°C and 127°C. The efficiency of the engine is _______.
a)
40%
b)
70%
c)
60%
d)
50%
|
|
Mahi Nair answered |
The Carnot cycle is a theoretical thermodynamic cycle that represents the most efficient engine possible. It consists of four reversible processes: two isothermal processes and two adiabatic processes.
The Carnot cycle can be represented on a temperature-entropy (T-s) diagram. The cycle starts at state A, where the working substance is in contact with a high-temperature reservoir at temperature T_h. In the first process, isothermal expansion (process AB), the working substance absorbs heat Q_h from the high-temperature reservoir, and its temperature remains constant at T_h. The working substance expands and does work on the surroundings.
Next, in the adiabatic expansion (process BC), the working substance does work on the surroundings without exchanging heat with the surroundings. As a result, the temperature of the working substance decreases from T_h to T_c.
In the third process, isothermal compression (process CD), the working substance is in contact with a low-temperature reservoir at temperature T_c. Heat Q_c is rejected to the low-temperature reservoir, and the temperature of the working substance remains constant at T_c. The working substance contracts and work is done on it by the surroundings.
Finally, in the adiabatic compression (process DA), the working substance is compressed without exchanging heat with the surroundings. The temperature of the working substance increases from T_c to T_h.
The Carnot cycle is reversible, meaning that it can be operated in reverse to act as a heat pump or refrigerator. The efficiency of the Carnot engine is given by:
Efficiency = 1 - (T_c / T_h)
Where T_c is the temperature of the low-temperature reservoir and T_h is the temperature of the high-temperature reservoir.
In the specific case you mentioned, the Carnot engine operates between temperatures T_h = 727 K and T_c = ??? (the temperature of the low-temperature reservoir is not specified). With this information, the efficiency of the Carnot engine can be calculated using the equation mentioned above.
The Carnot cycle can be represented on a temperature-entropy (T-s) diagram. The cycle starts at state A, where the working substance is in contact with a high-temperature reservoir at temperature T_h. In the first process, isothermal expansion (process AB), the working substance absorbs heat Q_h from the high-temperature reservoir, and its temperature remains constant at T_h. The working substance expands and does work on the surroundings.
Next, in the adiabatic expansion (process BC), the working substance does work on the surroundings without exchanging heat with the surroundings. As a result, the temperature of the working substance decreases from T_h to T_c.
In the third process, isothermal compression (process CD), the working substance is in contact with a low-temperature reservoir at temperature T_c. Heat Q_c is rejected to the low-temperature reservoir, and the temperature of the working substance remains constant at T_c. The working substance contracts and work is done on it by the surroundings.
Finally, in the adiabatic compression (process DA), the working substance is compressed without exchanging heat with the surroundings. The temperature of the working substance increases from T_c to T_h.
The Carnot cycle is reversible, meaning that it can be operated in reverse to act as a heat pump or refrigerator. The efficiency of the Carnot engine is given by:
Efficiency = 1 - (T_c / T_h)
Where T_c is the temperature of the low-temperature reservoir and T_h is the temperature of the high-temperature reservoir.
In the specific case you mentioned, the Carnot engine operates between temperatures T_h = 727 K and T_c = ??? (the temperature of the low-temperature reservoir is not specified). With this information, the efficiency of the Carnot engine can be calculated using the equation mentioned above.
Which among the following is the correct option for adiabatic reversible expansion of an ideal gas?- a)Entropy change for system > 0, Total entropy change = 0, ΔT = 0
- b)Enthalpy change = 0, Total entropy change = 0, Gibbs free energy change < 0>
- c)Internal energy change = 0, Entropy change for surroundings > 0, ΔQ = 0
- d)Entropy change for surroundings = 0, Total entropy change = 0, ΔT ≠ 0
Correct answer is option 'D'. Can you explain this answer?
Which among the following is the correct option for adiabatic reversible expansion of an ideal gas?
a)
Entropy change for system > 0, Total entropy change = 0, ΔT = 0
b)
Enthalpy change = 0, Total entropy change = 0, Gibbs free energy change < 0>
c)
Internal energy change = 0, Entropy change for surroundings > 0, ΔQ = 0
d)
Entropy change for surroundings = 0, Total entropy change = 0, ΔT ≠ 0

|
EduRev NEET answered |
For an adiabatic reversible expansion of an ideal gas, the correct option is:
Option D: Entropy change for surroundings = 0, Total entropy change = 0, ΔT ≠ 0
In an adiabatic process, there is no heat exchange with the surroundings, so the entropy change for the surroundings is zero. However, there is a change in temperature (ΔT) for the system. So, the correct answer is Option D.
Option D: Entropy change for surroundings = 0, Total entropy change = 0, ΔT ≠ 0
In an adiabatic process, there is no heat exchange with the surroundings, so the entropy change for the surroundings is zero. However, there is a change in temperature (ΔT) for the system. So, the correct answer is Option D.
The 37° C temperature is equal to nearly:- a)99.4° F
- b)98.6° F
- c)97.4° F
- d)100.4°F
Correct answer is option 'B'. Can you explain this answer?
a)
99.4° F
b)
98.6° F
c)
97.4° F
d)
100.4°F

|
Ciel Knowledge answered |
The temperature of 37°C is equal to nearly 98.6°F. So, the correct answer is Option B.
Railway lines, 25 m long, are being laid when the temperature is 15°C. Gaps of 1.8 cm are being left between them. If ? = 12×10^-6/°C, the temperature at which they will touch each other is ______- a)25°C
- b)50°C
- c)75°C
- d)100°C
Correct answer is option 'C'. Can you explain this answer?
a)
25°C
b)
50°C
c)
75°C
d)
100°C

|
Top Rankers answered |
To find the temperature at which the railway lines will touch each other, we can use the formula:
?T = (? * L * ?L) / (2 * L)
Where:
?T = Change in temperature
? = Coefficient of linear expansion
L = Original length of the railway lines
?L = Change in length (1.8 cm = 0.018 m)
?T = (12 × 10^-6/°C * 25 m * 0.018 m) / (2 * 25 m)
?T = 0.00027°C
The temperature change is 0.00027°C. To find the final temperature, we add this change to the initial temperature:
Final Temperature = Initial Temperature + ?T
Final Temperature = 15°C + 0.00027°C
Final Temperature ? 15.00027°C
So, the temperature at which the railway lines will touch each other is approximately 15.00027°C, which can be rounded to 15°C. Therefore, the correct answer is Option C.
?T = (? * L * ?L) / (2 * L)
Where:
?T = Change in temperature
? = Coefficient of linear expansion
L = Original length of the railway lines
?L = Change in length (1.8 cm = 0.018 m)
?T = (12 × 10^-6/°C * 25 m * 0.018 m) / (2 * 25 m)
?T = 0.00027°C
The temperature change is 0.00027°C. To find the final temperature, we add this change to the initial temperature:
Final Temperature = Initial Temperature + ?T
Final Temperature = 15°C + 0.00027°C
Final Temperature ? 15.00027°C
So, the temperature at which the railway lines will touch each other is approximately 15.00027°C, which can be rounded to 15°C. Therefore, the correct answer is Option C.
Thermal decomposition of limestone produces:- a)Quick lime
- b)Lime water
- c)Marble
- d)Slaked lime
Correct answer is option 'A'. Can you explain this answer?
a)
Quick lime
b)
Lime water
c)
Marble
d)
Slaked lime

|
Ambition Institute answered |
The thermal decomposition of limestone produces quicklime (calcium oxide). So, the correct answer is Option A.
In an isothermal process, the internal energy- a)increases
- b)decreases
- c)remains constant
- d)first increases then decreases
Correct answer is option 'C'. Can you explain this answer?
a)
increases
b)
decreases
c)
remains constant
d)
first increases then decreases
|
|
Rajesh Gupta answered |
In an isothermal process, the internal energy of a system remains constant. This means that the temperature of the system remains constant throughout the process, and there is no net change in the internal energy.
The change in internal energy of 2 moles of a gas is -10 J. Find the work done on the gas if the process is adiabatic.- a)20 J
- b)15 J
- c)10 J
- d)5 J
Correct answer is option 'C'. Can you explain this answer?
The change in internal energy of 2 moles of a gas is -10 J. Find the work done on the gas if the process is adiabatic.
a)
20 J
b)
15 J
c)
10 J
d)
5 J

|
Mohit Rajpoot answered |
In an adiabatic process, the change in internal energy (ΔU) is equal to the work done (W) on the gas. Given that ΔU = -10 J, the work done on the gas is also -10 J. Therefore, the correct answer is Option C.
A water drop is divided into 27 equal droplets. The pressure difference between the inner and outer side of the big drop will be:- a)Same as that for a smaller droplet
- b)1/3 of that for a smaller droplet
- c)1/6 of that for a smaller droplet
- d)Twice of that for a smaller droplet
Correct answer is option 'B'. Can you explain this answer?
a)
Same as that for a smaller droplet
b)
1/3 of that for a smaller droplet
c)
1/6 of that for a smaller droplet
d)
Twice of that for a smaller droplet

|
Stepway Academy answered |
When a water drop is divided into 27 equal droplets, the pressure difference between the inner and outer side of the big drop will be 1/3 of that for a smaller droplet. This is because the pressure inside a droplet is inversely proportional to its radius. So, the correct answer is Option B.
Identify Chiral Compound 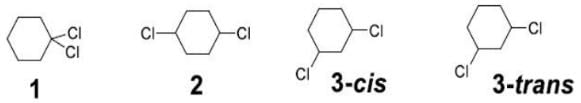
- a)one
- b)two
- c)three-trans
- d)three-cis
Correct answer is option 'C'. Can you explain this answer?
Identify Chiral Compound

a)
one
b)
two
c)
three-trans
d)
three-cis

|
Stepway Academy answered |
- The 1,1-dichloro isomer is omitted because it has no centers of chirality (symmetric carbon atom).
- In the case of cis & trans-1,4-dichlorocyclohexane, they do not have any chiral centers. Since in both the isomer, there will be a plane of symmetry (σ)
- containing the two Cl atoms, which will bisect the molecule into two identical halves.
- In Cis-1,3-dichlorocyclohexane, the two Cl atoms are identical and present in the cis orientation. The molecule possesses a plane of symmetry (σ) and is achiral.
- Because it is achiral even though it contains chirality centers it is called a meso compound.
- Trans-1,3-dimethylcyclohexane is an optically active compound and does not have a plane of symmetry. Thus, this is a chiral molecule.
- Trans-1,3-chlorocyclohexane is a chiral molecule. it is not superimposable on its mirror image.
- Trans-1,3-Dichlorocyclohexane has two chiral carbon atoms, and it lacks an internal plane of symmetry. It is not superimposable on its mirror image.
- It exists as a pair of optically active non-superimposable mirror-image isomers.
Chapter doubts & questions for September - Daily Test for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of September - Daily Test for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









