All Exams >
CDS >
General Awareness for CDS >
All Questions
All questions of Chemistry for CDS Exam
The ore of Aluminium is - a) Bauxite
- b) Chromium
- c) Mica
- d) Manganese
Correct answer is option 'A'. Can you explain this answer?
The ore of Aluminium is
a)
Bauxite
b)
Chromium
c)
Mica
d)
Manganese
|
|
Amit Kumar answered |
Bauxite, an aluminium ore, is the world’s main source of aluminium. It consists mostly of the minerals gibbsite.
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Which pair of molecules has the strongest dipole-dipole interactions?- a)NH3 and CH4
- b)CH4 and CH4
- c)CO3 and CO2
- d)NH3 and NH3
Correct answer is 'D'. Can you explain this answer?
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which pair of molecules has the strongest dipole-dipole interactions?
a)
NH3 and CH4
b)
CH4 and CH4
c)
CO3 and CO2
d)
NH3 and NH3
|
|
Naina Bansal answered |
NH3 and NH3. Both polar, asymmetric molecules. CH4 is nonpolar. CO2 is symetrical.
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of  Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is- a)London interaction
- b)dipole-dipole interaction
- c)hydrogen bonding
- d)dipole-induced dipole interaction
Correct answer is option 'A'. Can you explain this answer?
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
a)
London interaction
b)
dipole-dipole interaction
c)
hydrogen bonding
d)
dipole-induced dipole interaction
|
|
Rajeev Saxena answered |
(a) HF - Hydrogen bonding HCl,HBr,HI→ Dipole-dipole interaction, London-dispersion force.
The Defense Research Development Organisation (DRDO) has developed a drug named ‘Lukosin’. It will be used in the treatment of- a)Leukemia
- b)Lucoderma
- c)Lung cancer
- d)Brain tumor
Correct answer is option 'B'. Can you explain this answer?
The Defense Research Development Organisation (DRDO) has developed a drug named ‘Lukosin’. It will be used in the treatment of
a)
Leukemia
b)
Lucoderma
c)
Lung cancer
d)
Brain tumor

|
Rajdeep Roy answered |
Lukosin is a herbal drug developed by Defense Research Development Organisation(DRDO) for treatment of Lucoderma( White patches on skin).
In comparing gases with liquids , gases have ........ compressibility and...........density.- a)greater, smalle
- b)greater, greater
- c)smaller, smaller
- d)smaller, greater
Correct answer is option 'A'. Can you explain this answer?
In comparing gases with liquids , gases have ........ compressibility and...........density.
a)
greater, smalle
b)
greater, greater
c)
smaller, smaller
d)
smaller, greater
|
|
Neha Patel answered |
In a gas, the distance between molecules, whether monatomic or polyatomic, is very large compared with the size of the molecules; thus gases have a low density and are highly compressible.Density: The molecules of a liquid are packed relatively close together. Consequently, liquids are much denser than gases.
Hydrogen bonding reduces the quality of water molecules to
- a)repel
- b)attract
- c)compactly arrange
- d)slide over each other
Correct answer is option 'D'. Can you explain this answer?
Hydrogen bonding reduces the quality of water molecules to
a)
repel
b)
attract
c)
compactly arrange
d)
slide over each other
|
|
Shreya Gupta answered |
Hydrogen bonding is a type of attractive force that occurs between molecules when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In water molecules, hydrogen bonding occurs between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another water molecule. These hydrogen bonds cause the water molecules to attract each other and stick together, which gives water many of its unique properties, such as its high surface tension and its ability to act as a solvent. The hydrogen bonds do not cause the water molecules to repel each other or to compactly arrange, but they do make it more difficult for the molecules to slide over each other, which contributes to the high viscosity of water.
Which type of rocks in India produces manganese? - a) Gondwana
- b) Dharwar
- c) Vindhya
- d) Tertiary
Correct answer is option 'B'. Can you explain this answer?
Which type of rocks in India produces manganese?
a)
Gondwana
b)
Dharwar
c)
Vindhya
d)
Tertiary
|
|
Deepa Iyer answered |
The rocks of the Dharwar system are mainly sedimentary in origin and occur in narrow elongated synclines resting on the gneisses found in Bellary district, Mysore and the Aravallis of Rajputana. These rocks are enriched in manganese and iron ore which represents a significant resource of these metals.
Bishrampur is famous for the mining of - a) Copper ore
- b) Iron ore
- c) Coal
- d) Manganese
Correct answer is option 'C'. Can you explain this answer?
Bishrampur is famous for the mining of
a)
Copper ore
b)
Iron ore
c)
Coal
d)
Manganese
|
|
Vijay Kumar answered |
Chhattisgarh is the second most coal-producing state. It has 15% coal quantity of the country while it produces 16% coal in the country. The main coalfield of North Chhattisgarh includes Chirmiri, Kursia Bishrampur, Ghilmili, Sonhat, Lakhanpur Sendouorgarh and Ramkola.
Intermolecular forces can be out of the following.- a)van der Waais' forces
- b)Electrostatic forces existing between two oppositely charged ions
- c)Covalent bond between two like atoms
- d)Gravitational force
Correct answer is option 'A'. Can you explain this answer?
Intermolecular forces can be out of the following.
a)
van der Waais' forces
b)
Electrostatic forces existing between two oppositely charged ions
c)
Covalent bond between two like atoms
d)
Gravitational force
|
|
Shreya Gupta answered |
In molecular physics, the van der Waals forces, named after Dutch scientist Johannes Diderik van der Waals, are distance-dependent interactions between atoms or molecules. Unlike ionic or covalent bonds, these attractions are not a result of any chemical electronic bond, and they are comparatively weak and more susceptible to being perturbed. Van der Waals forces quickly vanish at longer distances between interacting molecules.
Van der Waals forces play a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. Van der Waals forces also define many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media.
Which one of the following pairs is not correctly matched?- a)Arjun : Indigenously produced Main Battle Tank (MBT)
- b)Phalcon : Cruise missile supplied by Russia to India.
- c)Saras : Indigenously developed civilian passenger aircraft.
- d)Operation Seabird: New Indian naval base at Karwar.
Correct answer is option 'B'. Can you explain this answer?
Which one of the following pairs is not correctly matched?
a)
Arjun : Indigenously produced Main Battle Tank (MBT)
b)
Phalcon : Cruise missile supplied by Russia to India.
c)
Saras : Indigenously developed civilian passenger aircraft.
d)
Operation Seabird: New Indian naval base at Karwar.

|
Juhi Patel answered |
Phalcon is radar system provided by Israel to India.
Which of the following elements replaced eka-Aluminium in Mendeleev's Periodic Table?- a)Scandium
- b)Gallium
- c)Titanium
- d)Germanium
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements replaced eka-Aluminium in Mendeleev's Periodic Table?
a)
Scandium
b)
Gallium
c)
Titanium
d)
Germanium
|
|
Sheikha Shah answered |
Answer :
Gallium
Gallium replaced eka-Aluminium in Mendeleev's Periodic Table.
The element used in the manufacture of safety matches is –- a)Phosphorous
- b)Magnesium
- c)Silicon
- d)Sulphur
Correct answer is option 'A'. Can you explain this answer?
The element used in the manufacture of safety matches is –
a)
Phosphorous
b)
Magnesium
c)
Silicon
d)
Sulphur
|
|
Dia Mehta answered |
One end of a match is coated with a material that can be ignited by frictional heat generated by striking the match against a suitable surface. The coated end of a match, known as the match "head," contains either phosphorus or phosphorus sesquisulfide as the active ingredient and gelatin as a binder.
Van der waals forces include the following except
- a)London forces
- b)dipole - dipole forces
- c)dipole- include dipole forces
- d)chemical bonding forces
Correct answer is option 'D'. Can you explain this answer?
Van der waals forces include the following except
a)
London forces
b)
dipole - dipole forces
c)
dipole- include dipole forces
d)
chemical bonding forces
|
|
Om Desai answered |
Chemical bonding forces are not considered to be part of van der Waals forces. Van der Waals forces include London forces, dipole-dipole forces, and dipole-induced dipole forces.
Diamond is harder than graphite because of –- a)difference in layers of atoms
- b)tetrahedral structure of diamond
- c)difference of crystalline structures
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Diamond is harder than graphite because of –
a)
difference in layers of atoms
b)
tetrahedral structure of diamond
c)
difference of crystalline structures
d)
None of these
|
|
Eshaan Kapoor answered |
Diamond is harder than graphite because diamond has a more complex structure. Diamond's structure is like many pentagons connected together, each pentagon sharing a side with another pentagon or each pentagon sharing a point with another pentagon. All the points are linked together in some way. Graphite's structure is very loose, with its bonds forming layers.
Which mineral is mined in Turamdih?- a) Kyanite
- b) Asbestos
- c) Mica
- d) Uranium
Correct answer is option 'D'. Can you explain this answer?
Which mineral is mined in Turamdih?
a)
Kyanite
b)
Asbestos
c)
Mica
d)
Uranium
|
|
Kavita Mehta answered |
- Turamdih Uranium deposit is located about 24 km west of Jaduguda.
Dipole-dipole interaction energy between stationary polar molecules is proportional to x and that between rotating molecules is proportional to y. Assume distance between polar molecules as r, then x and y are- a)
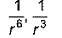
- b)
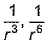
- c)
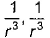
- d)
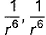
Correct answer is option 'B'. Can you explain this answer?
Dipole-dipole interaction energy between stationary polar molecules is proportional to x and that between rotating molecules is proportional to y. Assume distance between polar molecules as r, then x and y are
a)
b)
c)
d)

|
Ambition Institute answered |
Dipole-dipole interaction energy between stationary polar molecules is proportional to 1/ r3 and that between rotating polar molecules is proportional to 1/ r6 where ‘r’ is the distance between polar molecules
Besides dipole - dipole interaction, polar molecules can interact by London forces also.
Atom which must be present in hydrogen bonding is- a)hydrogen
- b)sodium
- c)calcium
- d)sulphur
Correct answer is option 'A'. Can you explain this answer?
Atom which must be present in hydrogen bonding is
a)
hydrogen
b)
sodium
c)
calcium
d)
sulphur
|
|
Nandini Patel answered |
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule. Usually the electronegative atom is oxygen, nitrogen, or fluorine, which has a partial negative charge. The hydrogen then has the partial positive charge.
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon- a)charge of interacting particles
- b)mass of interacting particles
- c)strength of permanent dipoles in the particles
- d)polarisability of interacting particles
Correct answer is option 'D'. Can you explain this answer?
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon
a)
charge of interacting particles
b)
mass of interacting particles
c)
strength of permanent dipoles in the particles
d)
polarisability of interacting particles
|
|
Rajat Kapoor answered |
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon. (d) strength of permanent dipoles in the particles.
Based on the following statements I and IS, select the correct answer from the codes given.Statement I Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules.Statement IIIntermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement il is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Based on the following statements I and IS, select the correct answer from the codes given.
Statement I
Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules.
Statement II
Intermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement il is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Geetika Shah answered |
Thermal energy is the energy of a body arising from motion of its atoms or molecules. It is directly proportional to the temperature of the substance. It is the measure of average kinetic energy of the particles of the matter and is thus responsible for movement of particles. This movement of particles is called thermal motion. We have already learnt that intermolecular forces tend to keep the molecules together but thermal energy of the molecules tends to keep them apart. Three states of matter are the result of balance between intermolecular forces and the thermal energy of the molecules.
Where do most of the known asteroids orbit the Sun?- a)Between the orbits of Mars and Jupiter
- b)Between the orbits of Venus and Earth
- c)Between the orbits of Earth and Mars
- d)Between the orbits of Pluto and Saturn
Correct answer is option 'A'. Can you explain this answer?
Where do most of the known asteroids orbit the Sun?
a)
Between the orbits of Mars and Jupiter
b)
Between the orbits of Venus and Earth
c)
Between the orbits of Earth and Mars
d)
Between the orbits of Pluto and Saturn

|
Deepika Gupta answered |
The asteroid belt is a doughnut-shaped concentration of many different sized asteroids between the orbits of Mars and Jupiter, closer to the orbit of Mars. The asteroids orbit the Sun. The asteroid belt is not smooth but there are concentric gaps in it known as Kirkwood gaps.
Param Padma is- a)a new civilian award in
- b)the name of supercomputer developed by India
- c)the name given to a proposed network of canals linking Northern and Southern rivers of India
- d)a software programme to facilitate e-governance in Madhya Pradesh
Correct answer is option 'B'. Can you explain this answer?
Param Padma is
a)
a new civilian award in
b)
the name of supercomputer developed by India
c)
the name given to a proposed network of canals linking Northern and Southern rivers of India
d)
a software programme to facilitate e-governance in Madhya Pradesh

|
Ipsita Bajaj answered |
Param Padma, a supercomputer was introduced in April 2003. It had a peak speed of 1024 GFLOPS (about 1 TFLOP) and a peak storage of 1 TB.
Urea is a -- a)Sodium fertilizer
- b)Phosphatic fertilizer
- c)Nitrogenous fertilizer
- d)Potassium fertilizer
Correct answer is option 'C'. Can you explain this answer?
Urea is a -
a)
Sodium fertilizer
b)
Phosphatic fertilizer
c)
Nitrogenous fertilizer
d)
Potassium fertilizer
|
|
Faizan Khan answered |
More than 90% of world production of urea is destined for use as a nitrogen-release fertilizer. Urea has the highest nitrogen content of all solid nitrogenous fertilizers in common use.
What is the most commonly used substance in fluorescent tubes?- a)Sodium oxide and argon
- b)Sodium vapour and neon
- c)Mercury vapour and argon
- d)Mercury oxide and neon
Correct answer is option 'C'. Can you explain this answer?
What is the most commonly used substance in fluorescent tubes?
a)
Sodium oxide and argon
b)
Sodium vapour and neon
c)
Mercury vapour and argon
d)
Mercury oxide and neon
|
|
Eshaan Kapoor answered |
A fluorescent lamp or fluorescent tube is a gas-discharge lamp that uses electricity to excite mercury vapour. it contains mercury vapour and argon. The excited mercury atoms produce short-wave ultraviolet light that then causes a phosphor to fluoresce, producing visible light. A fluorescent lamp converts electrical power into useful light more efficiently than an incandescent lamp.
Which district has become the first one in India to have high speed Rural Broadband Network?- a)Ajmer of Rajasthan
- b)Vidisha of Madhya Pradesh
- c)Idukki of Kerala
- d)Hisar of Haryana
Correct answer is option 'C'. Can you explain this answer?
Which district has become the first one in India to have high speed Rural Broadband Network?
a)
Ajmer of Rajasthan
b)
Vidisha of Madhya Pradesh
c)
Idukki of Kerala
d)
Hisar of Haryana

|
Asha Kumar answered |
Idukki district of Kerala has become first district in India to have high speed Rural Broadband Network i.e. National Optical Fibre Network (NOFN) Internet connectivity. NOFN is the largest rural connectivity project of its kind in the world.
Conversion of chemical energy into electrical energy occurs in -- a)Atomic bombs
- b)Dynamo
- c)A battery
- d)Electric heaters
Correct answer is option 'C'. Can you explain this answer?
Conversion of chemical energy into electrical energy occurs in -
a)
Atomic bombs
b)
Dynamo
c)
A battery
d)
Electric heaters
|
|
Alpana sengupta answered |
Conversion of Chemical Energy into Electrical Energy in a Battery
The correct answer to the question is option 'C' - a battery. A battery is a device that converts chemical energy into electrical energy through a chemical reaction. It consists of one or more electrochemical cells, which are composed of two electrodes (a cathode and an anode) and an electrolyte.
Chemical Reactions in a Battery:
When a battery is connected to an external circuit, a chemical reaction occurs within the battery. This chemical reaction involves the transfer of electrons from the anode to the cathode through the external circuit. The chemical reaction is typically a redox reaction (oxidation-reduction reaction), where one electrode undergoes oxidation (loses electrons) and the other undergoes reduction (gains electrons).
Here is a breakdown of the chemical reactions that occur in a typical battery:
1. Anode Reaction: At the anode, oxidation takes place, and the anode material loses electrons. For example, in a typical alkaline battery, the anode reaction is:
Zn(s) -> Zn2+(aq) + 2e-
In this reaction, zinc metal (Zn) is oxidized to form zinc ions (Zn2+) and release two electrons (2e-).
2. Cathode Reaction: At the cathode, reduction takes place, and the cathode material gains electrons. For example, in a typical alkaline battery, the cathode reaction is:
2MnO2(s) + H2O(l) + 2e- -> Mn2O3(s) + 2OH-(aq)
In this reaction, manganese dioxide (MnO2) is reduced by gaining two electrons (2e-) and reacts with water (H2O) to form manganese(III) oxide (Mn2O3) and hydroxide ions (OH-).
3. Overall Reaction: The overall reaction in a battery is the sum of the anode and cathode reactions. In the case of an alkaline battery, the overall reaction is:
Zn(s) + 2MnO2(s) + H2O(l) -> Zn2+(aq) + Mn2O3(s) + 2OH-(aq)
This overall reaction releases energy in the form of electrical potential energy, which can be harnessed to do work in an external circuit.
Conversion of Chemical Energy to Electrical Energy:
The conversion of chemical energy into electrical energy occurs due to the movement of electrons from the anode to the cathode through the external circuit. This flow of electrons creates an electric current, which can be used to power various devices or perform work.
The chemical reactions in the battery result in a potential difference, or voltage, between the anode and cathode. This potential difference drives the movement of electrons, creating an electric current. The electrical energy produced can then be used to power devices such as flashlights, remote controls, smartphones, and many more.
In summary, a battery converts chemical energy into electrical energy through a redox reaction. The anode undergoes oxidation, losing electrons, while the cathode undergoes reduction, gaining electrons. The movement of electrons through the external circuit creates an electric current, which can be used to power various devices.
The correct answer to the question is option 'C' - a battery. A battery is a device that converts chemical energy into electrical energy through a chemical reaction. It consists of one or more electrochemical cells, which are composed of two electrodes (a cathode and an anode) and an electrolyte.
Chemical Reactions in a Battery:
When a battery is connected to an external circuit, a chemical reaction occurs within the battery. This chemical reaction involves the transfer of electrons from the anode to the cathode through the external circuit. The chemical reaction is typically a redox reaction (oxidation-reduction reaction), where one electrode undergoes oxidation (loses electrons) and the other undergoes reduction (gains electrons).
Here is a breakdown of the chemical reactions that occur in a typical battery:
1. Anode Reaction: At the anode, oxidation takes place, and the anode material loses electrons. For example, in a typical alkaline battery, the anode reaction is:
Zn(s) -> Zn2+(aq) + 2e-
In this reaction, zinc metal (Zn) is oxidized to form zinc ions (Zn2+) and release two electrons (2e-).
2. Cathode Reaction: At the cathode, reduction takes place, and the cathode material gains electrons. For example, in a typical alkaline battery, the cathode reaction is:
2MnO2(s) + H2O(l) + 2e- -> Mn2O3(s) + 2OH-(aq)
In this reaction, manganese dioxide (MnO2) is reduced by gaining two electrons (2e-) and reacts with water (H2O) to form manganese(III) oxide (Mn2O3) and hydroxide ions (OH-).
3. Overall Reaction: The overall reaction in a battery is the sum of the anode and cathode reactions. In the case of an alkaline battery, the overall reaction is:
Zn(s) + 2MnO2(s) + H2O(l) -> Zn2+(aq) + Mn2O3(s) + 2OH-(aq)
This overall reaction releases energy in the form of electrical potential energy, which can be harnessed to do work in an external circuit.
Conversion of Chemical Energy to Electrical Energy:
The conversion of chemical energy into electrical energy occurs due to the movement of electrons from the anode to the cathode through the external circuit. This flow of electrons creates an electric current, which can be used to power various devices or perform work.
The chemical reactions in the battery result in a potential difference, or voltage, between the anode and cathode. This potential difference drives the movement of electrons, creating an electric current. The electrical energy produced can then be used to power devices such as flashlights, remote controls, smartphones, and many more.
In summary, a battery converts chemical energy into electrical energy through a redox reaction. The anode undergoes oxidation, losing electrons, while the cathode undergoes reduction, gaining electrons. The movement of electrons through the external circuit creates an electric current, which can be used to power various devices.
In which one of the following is a higher percentage of carbon found? - a) Lignite coal
- b) Peat coal
- c) Bituminous coal
- d) Anthracite coal
Correct answer is option 'D'. Can you explain this answer?
In which one of the following is a higher percentage of carbon found?
a)
Lignite coal
b)
Peat coal
c)
Bituminous coal
d)
Anthracite coal
|
|
Amit Kumar answered |
Anthracite has a higher percentage of Carbon. It has a carbon content of over 87% on a dry ash-free basis.
The gas used for filling weather balloons is –- a)helium
- b)hydrogen
- c)air
- d)nitrogen
Correct answer is option 'A'. Can you explain this answer?
The gas used for filling weather balloons is –
a)
helium
b)
hydrogen
c)
air
d)
nitrogen
|
|
Eshaan Kapoor answered |
A balloon is an inflatable flexible bag filled with a gas, such as helium, hydrogen, nitrous oxide, oxygen, or air. Modern balloons can be made from materials such as rubber, latex, polychloroprene, or a nylon fabric, while some early balloons were made of dried animal bladders, such as the pig bladder. Some balloons are used for decorative purposes, while others are used for practical purposes such as meteorology, medical treatment, military defense, or transportation.
Which metal is found at the following places-Hutti, Kolar and Ramgiri in India? - a) Aluminium
- b) Copper
- c) Silver
- d) Gold
Correct answer is option 'D'. Can you explain this answer?
Which metal is found at the following places-Hutti, Kolar and Ramgiri in India?
a)
Aluminium
b)
Copper
c)
Silver
d)
Gold
|
|
Anita Desai answered |
Gold is found in the Hutti, Kolar and Ramgiri regions of India.
What is Khetri in Rajasthan famous for?- a)Copper
- b)Mica
- c)Bauxite
- d)Limestone
Correct answer is option 'A'. Can you explain this answer?
What is Khetri in Rajasthan famous for?
a)
Copper
b)
Mica
c)
Bauxite
d)
Limestone
|
|
Arnav Malik answered |
Khetri, located in the Jhunjhunu district of Rajasthan, is famous for its copper mines. The region has a rich history of copper mining and has been a major source of copper production in India. Here is a detailed explanation of why Khetri is renowned for its copper:
Historical Significance:
- Khetri's association with copper mining dates back to ancient times. The region has been inhabited since the Indus Valley Civilization, and copper artifacts from that era have been discovered here.
- During the Mauryan period, Khetri was an important center for copper production. The excavation of ancient copper mines and smelting sites in the region suggests that copper mining was prevalent during that time.
- The Rajputs, who ruled the region during the medieval period, also recognized the significance of Khetri's copper mines and utilized them for their military and economic endeavors.
Abundance of Copper Deposits:
- Khetri is located in the Aravalli Range, which is known for its mineral-rich deposits. The region has abundant reserves of copper ore, making it an ideal location for mining activities.
- The copper deposits in Khetri are primarily found in the form of copper pyrite (chalcopyrite) and copper glance (chalcocite). These minerals contain a high concentration of copper, making them valuable for extraction.
Modern Copper Mining:
- With the advent of modern mining techniques, Khetri's copper mines witnessed significant development. The Hindustan Copper Limited (HCL), a public sector undertaking, has been instrumental in the extraction and processing of copper in Khetri.
- The Khetri Copper Complex, operated by HCL, consists of mines, concentrators, smelters, and refineries. It is one of the largest copper complexes in India and plays a crucial role in meeting the country's copper requirements.
Economic Importance:
- The copper mining industry in Khetri has contributed significantly to the economic development of the region and the state of Rajasthan.
- It has generated employment opportunities for the local population, both directly and indirectly. Many people are engaged in mining, transportation, and ancillary services related to the copper industry.
- The revenue generated from copper mining has contributed to the overall growth and infrastructure development of the region.
In conclusion, Khetri in Rajasthan is famous for its copper mines. The region's historical significance, abundance of copper deposits, modern mining techniques, and economic importance have collectively made Khetri a renowned hub for copper production in India.
Historical Significance:
- Khetri's association with copper mining dates back to ancient times. The region has been inhabited since the Indus Valley Civilization, and copper artifacts from that era have been discovered here.
- During the Mauryan period, Khetri was an important center for copper production. The excavation of ancient copper mines and smelting sites in the region suggests that copper mining was prevalent during that time.
- The Rajputs, who ruled the region during the medieval period, also recognized the significance of Khetri's copper mines and utilized them for their military and economic endeavors.
Abundance of Copper Deposits:
- Khetri is located in the Aravalli Range, which is known for its mineral-rich deposits. The region has abundant reserves of copper ore, making it an ideal location for mining activities.
- The copper deposits in Khetri are primarily found in the form of copper pyrite (chalcopyrite) and copper glance (chalcocite). These minerals contain a high concentration of copper, making them valuable for extraction.
Modern Copper Mining:
- With the advent of modern mining techniques, Khetri's copper mines witnessed significant development. The Hindustan Copper Limited (HCL), a public sector undertaking, has been instrumental in the extraction and processing of copper in Khetri.
- The Khetri Copper Complex, operated by HCL, consists of mines, concentrators, smelters, and refineries. It is one of the largest copper complexes in India and plays a crucial role in meeting the country's copper requirements.
Economic Importance:
- The copper mining industry in Khetri has contributed significantly to the economic development of the region and the state of Rajasthan.
- It has generated employment opportunities for the local population, both directly and indirectly. Many people are engaged in mining, transportation, and ancillary services related to the copper industry.
- The revenue generated from copper mining has contributed to the overall growth and infrastructure development of the region.
In conclusion, Khetri in Rajasthan is famous for its copper mines. The region's historical significance, abundance of copper deposits, modern mining techniques, and economic importance have collectively made Khetri a renowned hub for copper production in India.
Colour imparted to the Bunsen flame by strontium salt is -- a)bluish green
- b)apple-green
- c)brick red
- d)crimson red
Correct answer is option 'D'. Can you explain this answer?
Colour imparted to the Bunsen flame by strontium salt is -
a)
bluish green
b)
apple-green
c)
brick red
d)
crimson red
|
|
Aryan Khanna answered |
A flame test is performed by introducing a sample into the blue flame of a bunsen burner and noting any change in the colour of the flame. The tests can be used to detect the presence of some metallic elements in salts. With strontium salt, the colour of Bunsen flame ranges from crimson to red.
Percentage of carbon in steel ranges from –- a)0.1 to 1.5
- b)1.5 to 3.0
- c)3.0 to 4.0
- d)4.0 to 6.0
Correct answer is option 'A'. Can you explain this answer?
Percentage of carbon in steel ranges from –
a)
0.1 to 1.5
b)
1.5 to 3.0
c)
3.0 to 4.0
d)
4.0 to 6.0
|
|
Aryan Khanna answered |
Steel is an alloy made by combining iron and other elements, the most common of these being carbon. When carbon is used, its content in the steel is between 0.2% and 2. 1% by weight, depending on the grade. Other alloying elements sometimes used are manganese, chromium, vanadium and tungsten.
Which of the following is a super-cooled liquid?- a)Ice-cream
- b)Ammonia
- c)Glass
- d)Wood
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a super-cooled liquid?
a)
Ice-cream
b)
Ammonia
c)
Glass
d)
Wood
|
|
Faizan Khan answered |
Glass is considered to be a super - cooled liquid due to its lack of a first-order phase transition where certain thermodynamic variables such as volume, entropy and enthalpy are discontinuous through the glass transition. range. However, the glass transition may be described as analogous to a second-order phase transition where the intensive thermodynamic variables such as the thermal expansivity and heat capacity are discontinuous.
Jaduguda is famous for which of the following? - a) Iron-ore
- b) Manganese
- c) Gold
- d) Uranium
Correct answer is option 'D'. Can you explain this answer?
Jaduguda is famous for which of the following?
a)
Iron-ore
b)
Manganese
c)
Gold
d)
Uranium
|
|
Gauri Mukherjee answered |
Explanation:
Jaduguda is a small town located in the Singhbhum district of Jharkhand state in India. It is famous for its uranium mines which produce uranium ore, a key component in the production of nuclear energy.
Background:
India is one of the largest consumers of energy in the world, and with a rapidly growing population, the demand for energy is expected to increase significantly in the future. In order to meet this demand, India has been investing heavily in the development of nuclear energy as a clean and sustainable source of power.
Jaduguda is one of the most important uranium mining sites in India, and it is operated by the state-owned Uranium Corporation of India Limited (UCIL). The site was discovered in the late 1950s, and mining operations began in the 1960s. Since then, the site has been a major source of uranium for India's nuclear power plants.
Impact:
The uranium extracted from Jaduguda is used to fuel India's nuclear reactors, which generate electricity for millions of people. Nuclear power is considered to be a clean and efficient source of energy, and it is an important component of India's energy mix. The uranium mining operations in Jaduguda also provide employment and economic opportunities for the local community.
Environmental concerns:
Mining operations can have significant environmental impacts, and there have been concerns about the potential health and environmental risks associated with uranium mining in Jaduguda. The mining and processing of uranium ore can release radioactive materials into the environment, and there have been reports of contamination of groundwater and soil in the area. The UCIL has taken steps to address these concerns, such as implementing safety measures and monitoring the environmental impact of mining operations.
Conclusion:
Jaduguda's uranium mines are an important source of energy for India, and they provide employment and economic opportunities for the local community. However, there are also concerns about the potential environmental and health risks associated with uranium mining operations, and it is important to ensure that appropriate safety measures are in place to protect the environment and the local community.
Jaduguda is a small town located in the Singhbhum district of Jharkhand state in India. It is famous for its uranium mines which produce uranium ore, a key component in the production of nuclear energy.
Background:
India is one of the largest consumers of energy in the world, and with a rapidly growing population, the demand for energy is expected to increase significantly in the future. In order to meet this demand, India has been investing heavily in the development of nuclear energy as a clean and sustainable source of power.
Jaduguda is one of the most important uranium mining sites in India, and it is operated by the state-owned Uranium Corporation of India Limited (UCIL). The site was discovered in the late 1950s, and mining operations began in the 1960s. Since then, the site has been a major source of uranium for India's nuclear power plants.
Impact:
The uranium extracted from Jaduguda is used to fuel India's nuclear reactors, which generate electricity for millions of people. Nuclear power is considered to be a clean and efficient source of energy, and it is an important component of India's energy mix. The uranium mining operations in Jaduguda also provide employment and economic opportunities for the local community.
Environmental concerns:
Mining operations can have significant environmental impacts, and there have been concerns about the potential health and environmental risks associated with uranium mining in Jaduguda. The mining and processing of uranium ore can release radioactive materials into the environment, and there have been reports of contamination of groundwater and soil in the area. The UCIL has taken steps to address these concerns, such as implementing safety measures and monitoring the environmental impact of mining operations.
Conclusion:
Jaduguda's uranium mines are an important source of energy for India, and they provide employment and economic opportunities for the local community. However, there are also concerns about the potential environmental and health risks associated with uranium mining operations, and it is important to ensure that appropriate safety measures are in place to protect the environment and the local community.
Coking coal is a vital input in - a) Steelmaking
- b) Thermal power
- c) Copper smelting
- d) Sponge iron making
Correct answer is option 'A'. Can you explain this answer?
Coking coal is a vital input in
a)
Steelmaking
b)
Thermal power
c)
Copper smelting
d)
Sponge iron making
|
|
Jaya Chopra answered |
Coking coal is a vital input in Steelmaking
Coking coal, also known as metallurgical coal, is a crucial raw material in the steelmaking process. It plays a significant role in the production of high-quality steel. Let's delve into the details to understand why coking coal is essential in steelmaking.
1. Steelmaking Process:
- Steelmaking is the process of producing steel from iron ore and other materials.
- It involves the removal of impurities from iron ore to obtain pure iron.
- The pure iron is then mixed with various elements, such as carbon and alloying agents, to create different grades of steel.
- The mixture of iron ore and carbon is heated in a blast furnace to produce molten iron, which is further refined to remove impurities.
- Coking coal is used in the blast furnace as a fuel and a reducing agent to convert iron ore into molten iron.
2. Role of Coking Coal:
- Coking coal is primarily used to produce coke, a high-carbon fuel derived from coal.
- Coke acts as both a fuel and a reducing agent in the blast furnace.
- As a fuel, coke provides the necessary heat energy to sustain the high temperatures required for the steelmaking process.
- As a reducing agent, coke reacts with iron ore, reducing the oxygen content and transforming it into molten iron.
- The carbon content in coking coal is essential for these reactions to occur effectively.
- The coke produced from coking coal also helps in the removal of impurities, such as sulfur, during the steelmaking process.
3. Quality Requirements:
- The quality of coking coal is crucial for efficient steelmaking.
- It should have a high carbon content, low ash content, and low sulfur content.
- The carbon content provides the necessary heat energy and acts as a reducing agent.
- The low ash content ensures minimal impurities during the steelmaking process.
- The low sulfur content prevents the formation of sulfur compounds that can weaken the steel's mechanical properties.
Conclusion:
Coking coal is an indispensable input in the steelmaking industry. It serves as a fuel, a reducing agent, and helps remove impurities during the production of steel. The quality of coking coal directly impacts the efficiency and quality of the steelmaking process. Therefore, it is vital to have a consistent supply of high-quality coking coal for the steel industry to meet its demands and produce high-quality steel products.
Coking coal, also known as metallurgical coal, is a crucial raw material in the steelmaking process. It plays a significant role in the production of high-quality steel. Let's delve into the details to understand why coking coal is essential in steelmaking.
1. Steelmaking Process:
- Steelmaking is the process of producing steel from iron ore and other materials.
- It involves the removal of impurities from iron ore to obtain pure iron.
- The pure iron is then mixed with various elements, such as carbon and alloying agents, to create different grades of steel.
- The mixture of iron ore and carbon is heated in a blast furnace to produce molten iron, which is further refined to remove impurities.
- Coking coal is used in the blast furnace as a fuel and a reducing agent to convert iron ore into molten iron.
2. Role of Coking Coal:
- Coking coal is primarily used to produce coke, a high-carbon fuel derived from coal.
- Coke acts as both a fuel and a reducing agent in the blast furnace.
- As a fuel, coke provides the necessary heat energy to sustain the high temperatures required for the steelmaking process.
- As a reducing agent, coke reacts with iron ore, reducing the oxygen content and transforming it into molten iron.
- The carbon content in coking coal is essential for these reactions to occur effectively.
- The coke produced from coking coal also helps in the removal of impurities, such as sulfur, during the steelmaking process.
3. Quality Requirements:
- The quality of coking coal is crucial for efficient steelmaking.
- It should have a high carbon content, low ash content, and low sulfur content.
- The carbon content provides the necessary heat energy and acts as a reducing agent.
- The low ash content ensures minimal impurities during the steelmaking process.
- The low sulfur content prevents the formation of sulfur compounds that can weaken the steel's mechanical properties.
Conclusion:
Coking coal is an indispensable input in the steelmaking industry. It serves as a fuel, a reducing agent, and helps remove impurities during the production of steel. The quality of coking coal directly impacts the efficiency and quality of the steelmaking process. Therefore, it is vital to have a consistent supply of high-quality coking coal for the steel industry to meet its demands and produce high-quality steel products.
Consider the following statements—1. Balaghat is known for its diamond mines.2. Majhgaon is known for its manganese deposits.Which of the statements given above is/are correct?- a) 1 only
- b) 2 only
- c) Both 1 and 2
- d) Neither 1 nor 2
Correct answer is option 'D'. Can you explain this answer?
Consider the following statements—
1. Balaghat is known for its diamond mines.
2. Majhgaon is known for its manganese deposits.
Which of the statements given above is/are correct?
a)
1 only
b)
2 only
c)
Both 1 and 2
d)
Neither 1 nor 2
|
|
Vikram Verma answered |
Balaghat is known for its Manganese production. About 80% of Manganese production of the country comes from Balaghat. Majhgaon is known for its diamond mines, which is situated in the Panna district of Madhya Pradesh.
Which of the following is not present in German-silver?- a)Copper
- b)Nickel
- c)Silver
- d)Zinc
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not present in German-silver?
a)
Copper
b)
Nickel
c)
Silver
d)
Zinc
|
|
Dia Mehta answered |
Nickel silver, also known as German silver, Argentan, new silver, nickel brass, albata, alpacca, or electrum, is a copper alloy with nickel and often zinc. The usual formulation is 60% copper, 20% nickel and 20% zinc. Nickel silver is named for its silvery appearance, but it contains no elemental silver unless plated. The name "German silver" refers to its development by 19th century German metalworkers in imitation of the Chinese alloy known as paktong.
Enriched uranium is one in which?- a)Percentage of 235U has been artificially increased
- b)Percentage of U has been artificially increased
- c)Percentage of 234U has been artificially increased
- d)Extra energy is pumped from outside
Correct answer is option 'A'. Can you explain this answer?
Enriched uranium is one in which?
a)
Percentage of 235U has been artificially increased
b)
Percentage of U has been artificially increased
c)
Percentage of 234U has been artificially increased
d)
Extra energy is pumped from outside
|
|
Madhurima Tiwari answered |
Enriched uranium is one in which the percentage of 235U has been artificially increased.
Explanation:
Enriched uranium refers to a type of uranium in which the percentage of the isotope uranium-235 (235U) has been artificially increased. Uranium occurs naturally in the Earth's crust and is composed of three isotopes: uranium-238 (238U), uranium-235 (235U), and uranium-234 (234U). These isotopes have slightly different atomic masses due to variations in the number of neutrons in their nuclei.
Why is uranium enrichment necessary?
Uranium enrichment is necessary for several applications, including nuclear power generation and the production of nuclear weapons. Natural uranium as found in nature typically contains only about 0.7% uranium-235, while the majority is uranium-238. In order to use uranium as fuel in nuclear reactors or to create weapons-grade material, it is necessary to increase the concentration of uranium-235 through a process called enrichment.
The Uranium Enrichment Process:
The process of uranium enrichment involves increasing the concentration of uranium-235 by separating it from uranium-238. There are various methods of enrichment, but the most common one is centrifuge enrichment.
Centrifuge Enrichment:
In centrifuge enrichment, uranium hexafluoride (UF6) gas is fed into a series of high-speed centrifuges. These centrifuges spin at extremely high speeds, causing the heavier uranium-238 to migrate towards the outer edge while the lighter uranium-235 collects near the center. This separation is based on the slight difference in mass between the two isotopes.
Gradual Increase in Uranium-235 Concentration:
Through multiple stages of centrifuge enrichment, the concentration of uranium-235 can be gradually increased. The enriched uranium produced through this process is then used as fuel in nuclear power plants or can be further processed for military purposes.
Conclusion:
Enriched uranium is a type of uranium in which the percentage of uranium-235 has been artificially increased. This is achieved through a process called uranium enrichment, where the heavier uranium-238 is separated from the lighter uranium-235. Enriched uranium is used in various applications, including nuclear power generation and the production of nuclear weapons.
Explanation:
Enriched uranium refers to a type of uranium in which the percentage of the isotope uranium-235 (235U) has been artificially increased. Uranium occurs naturally in the Earth's crust and is composed of three isotopes: uranium-238 (238U), uranium-235 (235U), and uranium-234 (234U). These isotopes have slightly different atomic masses due to variations in the number of neutrons in their nuclei.
Why is uranium enrichment necessary?
Uranium enrichment is necessary for several applications, including nuclear power generation and the production of nuclear weapons. Natural uranium as found in nature typically contains only about 0.7% uranium-235, while the majority is uranium-238. In order to use uranium as fuel in nuclear reactors or to create weapons-grade material, it is necessary to increase the concentration of uranium-235 through a process called enrichment.
The Uranium Enrichment Process:
The process of uranium enrichment involves increasing the concentration of uranium-235 by separating it from uranium-238. There are various methods of enrichment, but the most common one is centrifuge enrichment.
Centrifuge Enrichment:
In centrifuge enrichment, uranium hexafluoride (UF6) gas is fed into a series of high-speed centrifuges. These centrifuges spin at extremely high speeds, causing the heavier uranium-238 to migrate towards the outer edge while the lighter uranium-235 collects near the center. This separation is based on the slight difference in mass between the two isotopes.
Gradual Increase in Uranium-235 Concentration:
Through multiple stages of centrifuge enrichment, the concentration of uranium-235 can be gradually increased. The enriched uranium produced through this process is then used as fuel in nuclear power plants or can be further processed for military purposes.
Conclusion:
Enriched uranium is a type of uranium in which the percentage of uranium-235 has been artificially increased. This is achieved through a process called uranium enrichment, where the heavier uranium-238 is separated from the lighter uranium-235. Enriched uranium is used in various applications, including nuclear power generation and the production of nuclear weapons.
Consider the following statements about GSLV Mk3
1. It can carry up to 4500 to 5000 kg satellites.
2. It is a 4 stage vehicle.
3. It will use cryogenic engine and not solid and liquid propellants.Q. Which of the above statements are correct?- a)1 only
- b)3 only
- c)2 and 3 only
- d)1, 2 and 3
Correct answer is option 'A'. Can you explain this answer?
Consider the following statements about GSLV Mk3
1. It can carry up to 4500 to 5000 kg satellites.
2. It is a 4 stage vehicle.
3. It will use cryogenic engine and not solid and liquid propellants.
1. It can carry up to 4500 to 5000 kg satellites.
2. It is a 4 stage vehicle.
3. It will use cryogenic engine and not solid and liquid propellants.
Q. Which of the above statements are correct?
a)
1 only
b)
3 only
c)
2 and 3 only
d)
1, 2 and 3
|
|
Dilshad Khan answered |
A
Consider the following statements
1. Thirty Meter Telescope will be the largest of the existing and announced extremely large telescopes (ELT).
2. The TMT will enable scientists to study fainter objects far away from earth providing information about early stages of the evolution of the universe.
3. India is also a partner in the project.Q. Which of the statements given above are correct?- a)1 and 2 only
- b)2 and 3 only
- c)1 and 3 only
- d)1, 2 and 3
Correct answer is option 'B'. Can you explain this answer?
Consider the following statements
1. Thirty Meter Telescope will be the largest of the existing and announced extremely large telescopes (ELT).
2. The TMT will enable scientists to study fainter objects far away from earth providing information about early stages of the evolution of the universe.
3. India is also a partner in the project.
1. Thirty Meter Telescope will be the largest of the existing and announced extremely large telescopes (ELT).
2. The TMT will enable scientists to study fainter objects far away from earth providing information about early stages of the evolution of the universe.
3. India is also a partner in the project.
Q. Which of the statements given above are correct?
a)
1 and 2 only
b)
2 and 3 only
c)
1 and 3 only
d)
1, 2 and 3

|
Bhargavi Singh answered |
Thirty Meter Telescope will be the second largest of the existing and announced extremely large telescopes (ELT) which is under construction on Mauna Kea in Hawaii. The TMT will enable scientists to study fainter objects far away from earth providing information about early stages of the evolution of the universe. As a founding member of the important international scientific project, India will be a 10 percent partner in the project and 70 percent of its contributions will be "in kind".
The pH of a neutral solution is -- a)0-7
- b)7
- c)7-14
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The pH of a neutral solution is -
a)
0-7
b)
7
c)
7-14
d)
None of the above
|
|
Dia Mehta answered |
The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, the solution turns more basic.
Commercial Vaseline is derived from -- a)plant gums
- b)coal tar
- c)wool wax
- d)petroleum
Correct answer is option 'D'. Can you explain this answer?
Commercial Vaseline is derived from -
a)
plant gums
b)
coal tar
c)
wool wax
d)
petroleum
|
|
Faizan Khan answered |
Vaseline is a brand of petroleum jelly based products owned by Anglo-Dutch company Unilever. While Vaseline can be used as a lubricant, it is also a useful moisture insulator for local skin conditions characterized by tissue dehydration. Vaseline helps protect minor cuts and burns.
Which of the following is a natural dye?- a)Crystal violet
- b)Aniline blue
- c)Alizarin
- d)Phenolphthalein
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a natural dye?
a)
Crystal violet
b)
Aniline blue
c)
Alizarin
d)
Phenolphthalein
|
|
Aryan Khanna answered |
Alizarin or 1,2-dihydroxyanthraquinone (also known as Mordant Red 11 and Turkey Red is an organic compound with formula C14H8O4 that has been used throughout history as a prominent red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus. In 1869, it became the first natural pigment to be duplicated synthetically.
What is "milk of magnesia" chemically?- a)Magnesium carbonate
- b)Sodium bicarbonate
- c)Calcium hydroxide
- d)Magnesium hydroxide
Correct answer is option 'D'. Can you explain this answer?
What is "milk of magnesia" chemically?
a)
Magnesium carbonate
b)
Sodium bicarbonate
c)
Calcium hydroxide
d)
Magnesium hydroxide
|
|
Anaya Patel answered |
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. As a suspension in water, it is often called milk of magnesia because of its milk-like appearance. The solid mineral form of magnesium hydroxide is known as brucite.
Detergents are –- a)Sodium salts of fatty acids
- b)Sodium salts of sulphonic acids
- c)Sodium salt of benzoic acid
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Detergents are –
a)
Sodium salts of fatty acids
b)
Sodium salts of sulphonic acids
c)
Sodium salt of benzoic acid
d)
None of the above
|
|
Shivani das answered |
Understanding Detergents
Detergents are substances used for cleaning and they play a vital role in various cleaning applications. They can be categorized based on their chemical structure and the type of surfactants they contain.
Types of Detergents
- Sodium Salts of Fatty Acids: These are traditional soaps made from natural fats and oils. They work effectively in soft water but may not perform well in hard water. Thus, they are not classified as synthetic detergents.
- Sodium Salts of Sulphonic Acids: This is the correct answer. Synthetic detergents, commonly used in household and industrial cleaning products, are derived from petrochemicals and often take the form of sodium salts of sulfonic acids. These detergents are effective in hard water, provide better cleaning power, and are designed to work in a variety of conditions.
- Sodium Salt of Benzoic Acid: While this compound can serve as a preservative in food, it is not classified as a detergent. It does not possess the surfactant properties necessary for cleaning applications.
Conclusion
The correct classification of detergents is essential to understand their functionality and application. Sodium salts of sulfonic acids are designed to provide enhanced cleaning capabilities, making option 'B' the correct choice. Their effectiveness in hard water and ability to emulsify oils and dirt are what set them apart from traditional soap-based cleaners.
Detergents are substances used for cleaning and they play a vital role in various cleaning applications. They can be categorized based on their chemical structure and the type of surfactants they contain.
Types of Detergents
- Sodium Salts of Fatty Acids: These are traditional soaps made from natural fats and oils. They work effectively in soft water but may not perform well in hard water. Thus, they are not classified as synthetic detergents.
- Sodium Salts of Sulphonic Acids: This is the correct answer. Synthetic detergents, commonly used in household and industrial cleaning products, are derived from petrochemicals and often take the form of sodium salts of sulfonic acids. These detergents are effective in hard water, provide better cleaning power, and are designed to work in a variety of conditions.
- Sodium Salt of Benzoic Acid: While this compound can serve as a preservative in food, it is not classified as a detergent. It does not possess the surfactant properties necessary for cleaning applications.
Conclusion
The correct classification of detergents is essential to understand their functionality and application. Sodium salts of sulfonic acids are designed to provide enhanced cleaning capabilities, making option 'B' the correct choice. Their effectiveness in hard water and ability to emulsify oils and dirt are what set them apart from traditional soap-based cleaners.
Which one amongst the following is not a Green House gas?- a)Nitrogen
- b)Carbon dioxide
- c)Carbon Monoxide
- d)Chloro fluoro carbons
Correct answer is option 'A'. Can you explain this answer?
Which one amongst the following is not a Green House gas?
a)
Nitrogen
b)
Carbon dioxide
c)
Carbon Monoxide
d)
Chloro fluoro carbons
|
|
Dia Mehta answered |
A greenhouse has (sometimes abbreviated GHG) is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. The primary greenhouse gases in the Earth's atmosphere are water vapour, carbon dioxide, methane, nitrous oxide, and ozone.
Manganite is an ore/mineral of ______.- a)Beryllium
- b)Chromium
- c)Manganese
- d)Copper
Correct answer is option 'C'. Can you explain this answer?
Manganite is an ore/mineral of ______.
a)
Beryllium
b)
Chromium
c)
Manganese
d)
Copper
|
|
Aryan Khanna answered |
Manganite is an ore mineral of manganese. As a manganese ore, it ranks after pyrolusite and romanechite.
Gobar gas mainly contains –- a)Carbon dioxide
- b)Carbon monoxide
- c)Hydrogen sulphide
- d)Methane
Correct answer is option 'D'. Can you explain this answer?
Gobar gas mainly contains –
a)
Carbon dioxide
b)
Carbon monoxide
c)
Hydrogen sulphide
d)
Methane
|
|
Eshaan Kapoor answered |
Biogas or Lobar gas is produced by the anaerobic digestion or fermentation of biodegradable materials such as biomass, manure, sewage, municipal waste, green waste, plant material, and crops. It comprises primarily methane (CH4) and carbon dioxide (CO2) and may have small amounts of hydrogen sulphide (H2S), moisture and siloxanes.
Ethanol containing 5% water is known as –- a)rectified spirit
- b)denatured spirit
- c)methylated alcohol
- d)power alcohol
Correct answer is option 'A'. Can you explain this answer?
Ethanol containing 5% water is known as –
a)
rectified spirit
b)
denatured spirit
c)
methylated alcohol
d)
power alcohol
|
|
Sayali nambiar answered |
Understanding Rectified Spirit
Rectified spirit refers to ethanol that has been purified through distillation, resulting in a high concentration of alcohol. When it contains 5% water, it is specifically known as rectified spirit.
Key Characteristics of Rectified Spirit:
- High Purity: Rectified spirit typically contains around 95% ethanol and 5% water, making it a highly concentrated form of alcohol.
- Uses: It is commonly used in laboratories, pharmaceuticals, and industries due to its effectiveness as a solvent and its ability to extract compounds.
- Distillation Process: The process involves distilling fermented liquids to separate alcohol from other components. This leads to the concentration of ethanol.
Comparison with Other Types of Alcohol:
- Denatured Spirit: This is ethanol that has been deliberately rendered undrinkable by adding toxic substances. It is used for industrial purposes and does not contain a specific percentage of water.
- Methylated Alcohol: This typically refers to methanol, which is highly toxic and not suitable for consumption.
- Power Alcohol: This term is used for ethanol blended with gasoline for use as fuel, usually containing more than just 5% water.
Conclusion:
The correct identification of ethanol with 5% water as rectified spirit highlights its purity and specific applications. Understanding these distinctions is crucial for fields such as chemistry and industry, making rectified spirit a valuable substance in both practical and scientific contexts.
Rectified spirit refers to ethanol that has been purified through distillation, resulting in a high concentration of alcohol. When it contains 5% water, it is specifically known as rectified spirit.
Key Characteristics of Rectified Spirit:
- High Purity: Rectified spirit typically contains around 95% ethanol and 5% water, making it a highly concentrated form of alcohol.
- Uses: It is commonly used in laboratories, pharmaceuticals, and industries due to its effectiveness as a solvent and its ability to extract compounds.
- Distillation Process: The process involves distilling fermented liquids to separate alcohol from other components. This leads to the concentration of ethanol.
Comparison with Other Types of Alcohol:
- Denatured Spirit: This is ethanol that has been deliberately rendered undrinkable by adding toxic substances. It is used for industrial purposes and does not contain a specific percentage of water.
- Methylated Alcohol: This typically refers to methanol, which is highly toxic and not suitable for consumption.
- Power Alcohol: This term is used for ethanol blended with gasoline for use as fuel, usually containing more than just 5% water.
Conclusion:
The correct identification of ethanol with 5% water as rectified spirit highlights its purity and specific applications. Understanding these distinctions is crucial for fields such as chemistry and industry, making rectified spirit a valuable substance in both practical and scientific contexts.
Gobar gas contains mainly –- a)methane
- b)ethylene
- c)propylene
- d)acetylene
Correct answer is option 'A'. Can you explain this answer?
Gobar gas contains mainly –
a)
methane
b)
ethylene
c)
propylene
d)
acetylene
|
|
Disha singhania answered |
Gobar gas, also known as biogas, contains mainly methane (CH4) and carbon dioxide (CO2). Methane is the primary component of biogas and is responsible for its combustible properties. Carbon dioxide is a byproduct of the anaerobic digestion process that produces biogas. In addition to methane and carbon dioxide, small amounts of other gases such as hydrogen sulfide (H2S), nitrogen (N2), and trace amounts of various impurities may also be present in gobar gas.
The Refrigerant 'FREON' is –- a)Calcium Tetra Fluoride
- b)Difluoro Dichloro Methane
- c)Fluorspar and Felspar
- d)Hydrofluosilicic Acid
Correct answer is option 'B'. Can you explain this answer?
The Refrigerant 'FREON' is –
a)
Calcium Tetra Fluoride
b)
Difluoro Dichloro Methane
c)
Fluorspar and Felspar
d)
Hydrofluosilicic Acid
|
|
Eshaan Kapoor answered |
Dichlorodifluorornethane (R-12), is a colourless gas, and usually sold under the brand name Freon-12, is a chlorofluorocarbon halornethane (CFC), used as a refrigrant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in the United States along with many other countries in 1994 clue to concerns about damage to the ozone layer. It is soluble in many organic solvents.
Chapter doubts & questions for Chemistry - General Awareness for CDS 2025 is part of CDS exam preparation. The chapters have been prepared according to the CDS exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for CDS 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemistry - General Awareness for CDS in English & Hindi are available as part of CDS exam.
Download more important topics, notes, lectures and mock test series for CDS Exam by signing up for free.
General Awareness for CDS
228 videos|314 docs|68 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









