All Exams >
Grade 2 >
Science for Grade 2 >
All Questions
All questions of Materials Can Change for Grade 2 Exam
Plastic is a:- a)Natural material
- b)Man-made material
- c)Living thing
- d)All of the above
Correct answer is option 'B'. Can you explain this answer?
Plastic is a:
a)
Natural material
b)
Man-made material
c)
Living thing
d)
All of the above
|
|
Sara singh answered |
Man-made material
Plastic is a man-made material, meaning it is not found in nature but is instead created by humans through chemical processes. Here are some key points to explain why plastic is considered a man-made material:
Composition:
Plastic is composed of synthetic polymers, which are large molecules made up of repeating units called monomers. These polymers are created through the combination of various chemicals derived from fossil fuels like oil and natural gas.
Manufacturing:
Plastic is manufactured in factories through processes like polymerization, extrusion, and molding. These processes involve heating and shaping the raw materials to create the desired plastic products.
Versatility:
One of the main advantages of plastic is its versatility, as it can be molded into different shapes and forms to serve a wide range of purposes. This adaptability is a result of the man-made nature of plastic.
Environmental impact:
The man-made nature of plastic also contributes to its environmental impact, as many types of plastic are not biodegradable and can persist in the environment for hundreds of years. This has led to concerns about plastic pollution and its effects on ecosystems and wildlife.
In conclusion, plastic is considered a man-made material because it is created by humans through chemical processes, as opposed to being a natural material like wood or cotton. Its composition, manufacturing processes, versatility, and environmental impact all reflect its status as a man-made material.
Plastic is a man-made material, meaning it is not found in nature but is instead created by humans through chemical processes. Here are some key points to explain why plastic is considered a man-made material:
Composition:
Plastic is composed of synthetic polymers, which are large molecules made up of repeating units called monomers. These polymers are created through the combination of various chemicals derived from fossil fuels like oil and natural gas.
Manufacturing:
Plastic is manufactured in factories through processes like polymerization, extrusion, and molding. These processes involve heating and shaping the raw materials to create the desired plastic products.
Versatility:
One of the main advantages of plastic is its versatility, as it can be molded into different shapes and forms to serve a wide range of purposes. This adaptability is a result of the man-made nature of plastic.
Environmental impact:
The man-made nature of plastic also contributes to its environmental impact, as many types of plastic are not biodegradable and can persist in the environment for hundreds of years. This has led to concerns about plastic pollution and its effects on ecosystems and wildlife.
In conclusion, plastic is considered a man-made material because it is created by humans through chemical processes, as opposed to being a natural material like wood or cotton. Its composition, manufacturing processes, versatility, and environmental impact all reflect its status as a man-made material.
Among the following substances, which shows three states of matter?- a)Balloon
- b)Water
- c)Butter
- d)Oil
Correct answer is option 'B'. Can you explain this answer?
Among the following substances, which shows three states of matter?
a)
Balloon
b)
Water
c)
Butter
d)
Oil

|
Rajveer Sonawane answered |
Water is the following substances ,which shows three states of matter
1)Solid - Ice
2) Liquid - Water
3) Gas - Vapour
1)Solid - Ice
2) Liquid - Water
3) Gas - Vapour
Which is a set of transparent materials ?- a)Glass and air
- b)Water and glass
- c)Water and air
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
Which is a set of transparent materials ?
a)
Glass and air
b)
Water and glass
c)
Water and air
d)
All of the above
|
|
Sarika dubey answered |
Transparent Materials:
Glass and air, water and glass, and water and air are all examples of transparent materials.
Glass and Air:
Glass is a transparent material that allows light to pass through it. Air is also a transparent material. When light passes through both glass and air, it continues on its path without being significantly absorbed or scattered.
Water and Glass:
Water is another transparent material that allows light to pass through it. Glass, like water, is also a transparent material. When light passes through water and glass, it also continues on its path without being significantly absorbed or scattered.
Water and Air:
Water and air are both transparent materials that allow light to pass through them. When light passes through water and air, it continues on its path without being significantly absorbed or scattered.
Conclusion:
Therefore, glass and air, water and glass, and water and air are all sets of transparent materials. They allow light to pass through them, making them suitable for various applications where transparency is required.
Glass and air, water and glass, and water and air are all examples of transparent materials.
Glass and Air:
Glass is a transparent material that allows light to pass through it. Air is also a transparent material. When light passes through both glass and air, it continues on its path without being significantly absorbed or scattered.
Water and Glass:
Water is another transparent material that allows light to pass through it. Glass, like water, is also a transparent material. When light passes through water and glass, it also continues on its path without being significantly absorbed or scattered.
Water and Air:
Water and air are both transparent materials that allow light to pass through them. When light passes through water and air, it continues on its path without being significantly absorbed or scattered.
Conclusion:
Therefore, glass and air, water and glass, and water and air are all sets of transparent materials. They allow light to pass through them, making them suitable for various applications where transparency is required.
Amit wants to drink Lemonade. He added Lemon, sugar in water and mixed it well. Now prepared the Lemonade is a- a)Soli
- b)Liquid
- c)Gas
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
Amit wants to drink Lemonade. He added Lemon, sugar in water and mixed it well. Now prepared the Lemonade is a
a)
Soli
b)
Liquid
c)
Gas
d)
All of these
|
|
Atharva Ghoshal answered |
Lemonade: A Mixture of Liquids
When Amit prepares lemonade, he combines several ingredients: lemon juice, sugar, and water. Let's explore why the correct answer is "Liquid."
What is Lemonade?
- Lemonade is primarily a liquid drink.
- It consists mainly of water, which is the solvent in this mixture.
- Lemon juice and sugar dissolve in the water, enhancing the flavor.
Components of Lemonade
- Lemon: Provides citric acid and flavor. It is a liquid when juiced.
- Sugar: Dissolves in water, making the solution sweet. It does not exist as a distinct phase in the drink.
- Water: The primary component, which makes lemonade a liquid. It acts as the base for the other ingredients.
Why Not Solid or Gas?
- Solid: While sugar is a solid before mixing, it dissolves in the water and does not remain as a solid in the prepared lemonade.
- Gas: Lemonade can have bubbles if carbonated, but the primary state of the drink is still liquid. The bubbles (gas) are not the main component.
Conclusion
Therefore, the prepared lemonade is classified as a liquid, as it is a homogeneous mixture where the ingredients are dissolved and distributed evenly. The correct answer is indeed option 'B': Liquid.
When Amit prepares lemonade, he combines several ingredients: lemon juice, sugar, and water. Let's explore why the correct answer is "Liquid."
What is Lemonade?
- Lemonade is primarily a liquid drink.
- It consists mainly of water, which is the solvent in this mixture.
- Lemon juice and sugar dissolve in the water, enhancing the flavor.
Components of Lemonade
- Lemon: Provides citric acid and flavor. It is a liquid when juiced.
- Sugar: Dissolves in water, making the solution sweet. It does not exist as a distinct phase in the drink.
- Water: The primary component, which makes lemonade a liquid. It acts as the base for the other ingredients.
Why Not Solid or Gas?
- Solid: While sugar is a solid before mixing, it dissolves in the water and does not remain as a solid in the prepared lemonade.
- Gas: Lemonade can have bubbles if carbonated, but the primary state of the drink is still liquid. The bubbles (gas) are not the main component.
Conclusion
Therefore, the prepared lemonade is classified as a liquid, as it is a homogeneous mixture where the ingredients are dissolved and distributed evenly. The correct answer is indeed option 'B': Liquid.
Which of the following is not filled with air?- a)Football
- b)Lemon
- c)Balloon
- d)Tyre
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not filled with air?
a)
Football
b)
Lemon
c)
Balloon
d)
Tyre
|
|
Swati Sharma answered |
Lemon is not filled with air.
State True and False and choose the correct option(i) Liquids on heating changes into vapour.(ii) Air does not occupy any space.(iii) In solids, molecules are packed and closed together.(iv) Water is a mixture of different elements.- a)TFTF
- b)TTFF
- c)FTFT
- d)FFTT
Correct answer is option 'A'. Can you explain this answer?
State True and False and choose the correct option
(i) Liquids on heating changes into vapour.
(ii) Air does not occupy any space.
(iii) In solids, molecules are packed and closed together.
(iv) Water is a mixture of different elements.
a)
TFTF
b)
TTFF
c)
FTFT
d)
FFTT
|
|
Gitanjali Nair answered |
True and False statements:
(i) Liquids on heating changes into vapour. - True
(ii) Air does not occupy any space. - False
(iii) In solids, molecules are packed and closed together. - True
(iv) Water is a mixture of different elements. - False
Explanation:
(i) Liquids on heating changes into vapour.
This statement is true. When a liquid is heated, it gains energy and the kinetic energy of its molecules increases. As a result, the molecules start moving faster and overcome the intermolecular forces holding them together. This leads to the liquid changing into its gaseous state, which is called vaporization or evaporation.
(ii) Air does not occupy any space.
This statement is false. Air, like any other gas, occupies space. It is made up of molecules and particles that have mass and volume. These molecules move freely and fill the space available to them. For example, if you blow up a balloon, the air molecules inside the balloon occupy the space inside it.
(iii) In solids, molecules are packed and closed together.
This statement is true. In solids, the molecules are tightly packed and closely arranged. The intermolecular forces between the molecules are strong, which keeps them in a fixed position. This is why solids have a definite shape and volume.
(iv) Water is a mixture of different elements.
This statement is false. Water is not a mixture of different elements. It is a compound made up of two elements, hydrogen and oxygen. The chemical formula of water is H2O, which means it consists of two hydrogen atoms bonded to one oxygen atom.
Correct option: The correct option is (a) TFTF.
(i) Liquids on heating changes into vapour. - True
(ii) Air does not occupy any space. - False
(iii) In solids, molecules are packed and closed together. - True
(iv) Water is a mixture of different elements. - False
Explanation:
(i) Liquids on heating changes into vapour.
This statement is true. When a liquid is heated, it gains energy and the kinetic energy of its molecules increases. As a result, the molecules start moving faster and overcome the intermolecular forces holding them together. This leads to the liquid changing into its gaseous state, which is called vaporization or evaporation.
(ii) Air does not occupy any space.
This statement is false. Air, like any other gas, occupies space. It is made up of molecules and particles that have mass and volume. These molecules move freely and fill the space available to them. For example, if you blow up a balloon, the air molecules inside the balloon occupy the space inside it.
(iii) In solids, molecules are packed and closed together.
This statement is true. In solids, the molecules are tightly packed and closely arranged. The intermolecular forces between the molecules are strong, which keeps them in a fixed position. This is why solids have a definite shape and volume.
(iv) Water is a mixture of different elements.
This statement is false. Water is not a mixture of different elements. It is a compound made up of two elements, hydrogen and oxygen. The chemical formula of water is H2O, which means it consists of two hydrogen atoms bonded to one oxygen atom.
Correct option: The correct option is (a) TFTF.
Toothbrushes are made from what material?- a)Steel
- b)Plastic
- c)Water
- d)Cotton
Correct answer is option 'B'. Can you explain this answer?
Toothbrushes are made from what material?
a)
Steel
b)
Plastic
c)
Water
d)
Cotton
|
|
Swati Sharma answered |
Toothbrushes are made from plastic.
Ball can be best, if made up of- a)Cotton
- b)Plastic
- c)Rubber
- d)Metal
Correct answer is option 'C'. Can you explain this answer?
Ball can be best, if made up of
a)
Cotton
b)
Plastic
c)
Rubber
d)
Metal
|
|
Rahul Verma answered |
Rubber ball is best because it is light in weight and bouncy.
The process by which solid directly changes into vapour- a)Condensation
- b)Sublimation
- c)Melting
- d)Freezing
Correct answer is option 'B'. Can you explain this answer?
The process by which solid directly changes into vapour
a)
Condensation
b)
Sublimation
c)
Melting
d)
Freezing
|
|
Rahul Verma answered |
Sublimation is the process when a solid is heated and it directly changes into its gaseous form without changing into liquid state.
An oily thin paper sheet will be __.- a)transparent
- b)translucent
- c)opaque
- d)cannot be predicted
Correct answer is option 'B'. Can you explain this answer?
An oily thin paper sheet will be __.
a)
transparent
b)
translucent
c)
opaque
d)
cannot be predicted
|
|
Sarika dubey answered |
Answer:
Introduction:
In this question, we are asked to determine the property of an oily thin paper sheet. The options given are:
a) transparent
b) translucent
c) opaque
d) cannot be predicted
Explanation:
To determine the property of the oily thin paper sheet, we need to understand the terms transparent, translucent, and opaque.
Transparent:
- Transparent materials allow light to pass through them easily, without scattering the light.
- When an object is transparent, we are able to see objects clearly through it.
- Examples of transparent materials include glass, clear plastic, and air.
Translucent:
- Translucent materials allow some light to pass through them, but they also scatter the light.
- When an object is translucent, we can see shapes and shadows through it, but not clear details.
- Examples of translucent materials include frosted glass, wax paper, and some plastics.
Opaque:
- Opaque materials do not allow light to pass through them.
- When an object is opaque, we cannot see through it at all.
- Examples of opaque materials include wood, metal, and cardboard.
Analysis:
Based on the given options, we can eliminate options a) transparent and c) opaque because an oily thin paper sheet is not transparent like glass or plastic, nor is it opaque like wood or metal.
Conclusion:
Therefore, the correct answer is option b) translucent. An oily thin paper sheet allows some light to pass through it, but it also scatters the light, making it translucent.
Introduction:
In this question, we are asked to determine the property of an oily thin paper sheet. The options given are:
a) transparent
b) translucent
c) opaque
d) cannot be predicted
Explanation:
To determine the property of the oily thin paper sheet, we need to understand the terms transparent, translucent, and opaque.
Transparent:
- Transparent materials allow light to pass through them easily, without scattering the light.
- When an object is transparent, we are able to see objects clearly through it.
- Examples of transparent materials include glass, clear plastic, and air.
Translucent:
- Translucent materials allow some light to pass through them, but they also scatter the light.
- When an object is translucent, we can see shapes and shadows through it, but not clear details.
- Examples of translucent materials include frosted glass, wax paper, and some plastics.
Opaque:
- Opaque materials do not allow light to pass through them.
- When an object is opaque, we cannot see through it at all.
- Examples of opaque materials include wood, metal, and cardboard.
Analysis:
Based on the given options, we can eliminate options a) transparent and c) opaque because an oily thin paper sheet is not transparent like glass or plastic, nor is it opaque like wood or metal.
Conclusion:
Therefore, the correct answer is option b) translucent. An oily thin paper sheet allows some light to pass through it, but it also scatters the light, making it translucent.
Which of the following material is used to make lunch boxes?
- a)Glass
- b)Paper
- c)Plastic
- d)Wax
Correct answer is option 'C'. Can you explain this answer?
Which of the following material is used to make lunch boxes?
a)
Glass
b)
Paper
c)
Plastic
d)
Wax
|
|
Swati Sharma answered |
Plastic is used to make lunch boxes.
Air is a mixture of ______.
- a)Solids
- b)Liquids
- c)Gases
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Air is a mixture of ______.
a)
Solids
b)
Liquids
c)
Gases
d)
None of these
|
|
Ameya Singh answered |
Explanation:
Introduction:
Air is the mixture of gases that surrounds the Earth. It is essential for the survival of all living organisms, including humans. This mixture consists of various gases, such as nitrogen, oxygen, carbon dioxide, and traces of other gases.
Composition of Air:
Air is primarily composed of three main gases: nitrogen, oxygen, and small amounts of other gases. The composition of air is as follows:
- Nitrogen: It constitutes about 78% of the total air volume. Nitrogen is an odorless and colorless gas that is essential for the growth of plants and animals.
- Oxygen: It makes up approximately 21% of the air volume. Oxygen is crucial for the respiration process in living organisms, as it is used to release energy from food.
- Carbon Dioxide: It makes up only about 0.04% of the air volume. Carbon dioxide is a greenhouse gas that plays a vital role in regulating the Earth's temperature.
- Other Gases: Besides nitrogen, oxygen, and carbon dioxide, air also contains traces of gases like argon, neon, helium, methane, and ozone.
Properties of Gases:
Gases have certain characteristics that distinguish them from solids and liquids:
- Gases have no fixed shape or volume. They take the shape and volume of the container they are in.
- Gases are compressible. This means that their volume can be reduced by applying pressure.
- Gases have low density compared to solids and liquids.
- Gases can easily mix with other gases, leading to the homogeneous nature of air.
Air as a Mixture of Gases:
Based on the properties of gases and the composition of air, it is evident that air is a mixture of gases:
- Air does not have a fixed shape or volume. It takes the shape and volume of its container, just like gases.
- Air is a homogeneous mixture, meaning that it has a uniform composition throughout. This is because the gases in air mix thoroughly with each other.
- Air can be separated into its constituent gases through various processes, such as fractional distillation or filtration.
Conclusion:
In conclusion, air is a mixture of gases. It consists of primarily nitrogen, oxygen, carbon dioxide, and traces of other gases. The properties of gases and the composition of air support the fact that air is a mixture of gases.
Introduction:
Air is the mixture of gases that surrounds the Earth. It is essential for the survival of all living organisms, including humans. This mixture consists of various gases, such as nitrogen, oxygen, carbon dioxide, and traces of other gases.
Composition of Air:
Air is primarily composed of three main gases: nitrogen, oxygen, and small amounts of other gases. The composition of air is as follows:
- Nitrogen: It constitutes about 78% of the total air volume. Nitrogen is an odorless and colorless gas that is essential for the growth of plants and animals.
- Oxygen: It makes up approximately 21% of the air volume. Oxygen is crucial for the respiration process in living organisms, as it is used to release energy from food.
- Carbon Dioxide: It makes up only about 0.04% of the air volume. Carbon dioxide is a greenhouse gas that plays a vital role in regulating the Earth's temperature.
- Other Gases: Besides nitrogen, oxygen, and carbon dioxide, air also contains traces of gases like argon, neon, helium, methane, and ozone.
Properties of Gases:
Gases have certain characteristics that distinguish them from solids and liquids:
- Gases have no fixed shape or volume. They take the shape and volume of the container they are in.
- Gases are compressible. This means that their volume can be reduced by applying pressure.
- Gases have low density compared to solids and liquids.
- Gases can easily mix with other gases, leading to the homogeneous nature of air.
Air as a Mixture of Gases:
Based on the properties of gases and the composition of air, it is evident that air is a mixture of gases:
- Air does not have a fixed shape or volume. It takes the shape and volume of its container, just like gases.
- Air is a homogeneous mixture, meaning that it has a uniform composition throughout. This is because the gases in air mix thoroughly with each other.
- Air can be separated into its constituent gases through various processes, such as fractional distillation or filtration.
Conclusion:
In conclusion, air is a mixture of gases. It consists of primarily nitrogen, oxygen, carbon dioxide, and traces of other gases. The properties of gases and the composition of air support the fact that air is a mixture of gases.
The materials from which the light can pass through it- a)Opaque
- b)Strong
- c)Waterproof
- d)Transparent
Correct answer is option 'D'. Can you explain this answer?
The materials from which the light can pass through it
a)
Opaque
b)
Strong
c)
Waterproof
d)
Transparent
|
|
Rahul Verma answered |
Transparent materials are the substances through which light can pass.
State True and False and choose the correct option(i) The process in which liquid changes into solid is called melting.(ii) Gases are hard to compress.(iii) Condensation is the change of Gas into liquid.(iv) Liquids can take the shape of a container.- a)FTFT
- b)TFTF
- c)TTFF
- d)FFTT
Correct answer is option 'D'. Can you explain this answer?
State True and False and choose the correct option
(i) The process in which liquid changes into solid is called melting.
(ii) Gases are hard to compress.
(iii) Condensation is the change of Gas into liquid.
(iv) Liquids can take the shape of a container.
a)
FTFT
b)
TFTF
c)
TTFF
d)
FFTT
|
|
Rahul Verma answered |
The process in which liquid changes into solid is called Freezing. Gases are easily compressible.
Mark an odd one out.- a)Shoes
- b)Coffee
- c)Buttermilk
- d)Tea
Correct answer is option 'A'. Can you explain this answer?
Mark an odd one out.
a)
Shoes
b)
Coffee
c)
Buttermilk
d)
Tea
|
|
Rahul Verma answered |
Coffee, Buttermilk and Tea are liquids whereas Shoe is a solid.
Which among the following shows Sublimation?- a)Petrol
- b)Camphor
- c)Coal
- d)Paper
Correct answer is option 'B'. Can you explain this answer?
Which among the following shows Sublimation?
a)
Petrol
b)
Camphor
c)
Coal
d)
Paper
|
|
Rahul Verma answered |
Camphor is a solid substance which on heating changes into gaseous state without changing into liquid state.
500 cm3 of air can be put in which of these containers?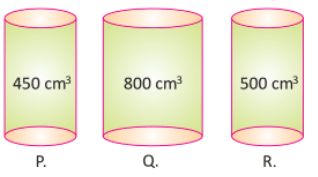
- a)Only R
- b)Only Q and R
- c)Only Q
- d)P, Q and R
Correct answer is option 'B'. Can you explain this answer?
500 cm3 of air can be put in which of these containers?

a)
Only R
b)
Only Q and R
c)
Only Q
d)
P, Q and R
|
|
Rahul Verma answered |
500 cm³ of air can be put in containers Q and R. The molecules of gas are loosely packed, so it can flow easily and can spread into the container.
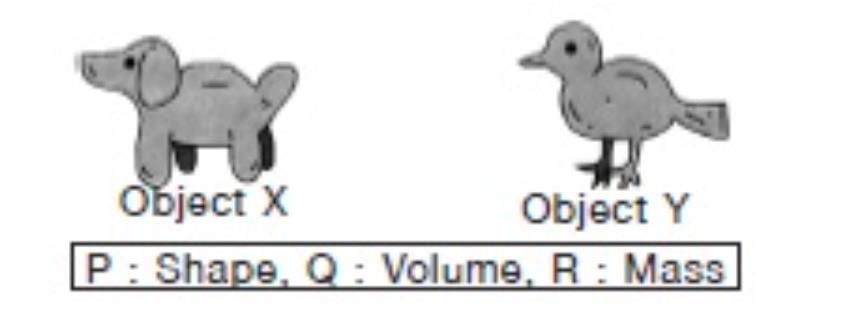
Siddhi took some plasticine and shaped it into object X. She reshaped the same piece of plasticine into object Y. We can conclude that both objects have the same………… .
- a)P and Q only
- b)P and R only
- c)Q and R only
- d)P, Q and R
Correct answer is option 'C'. Can you explain this answer?
Siddhi took some plasticine and shaped it into object X. She reshaped the same piece of plasticine into object Y. We can conclude that both objects have the same………… .

a)
P and Q only
b)
P and R only
c)
Q and R only
d)
P, Q and R
|
|
Rahul Verma answered |
The volume and the mass of the plasticine remains the same, but the shape of both the objects are different.
Which of the following is/are made up of one material only?- a)

- b)

- c)

- d)Both (a) and (c)
Correct answer is option 'D'. Can you explain this answer?
Which of the following is/are made up of one material only?
a)

b)

c)

d)
Both (a) and (c)

|
Dr Manju Sen answered |
Glass and scarf are made up of one type of material only.
Select the INCORRECT statement.- a)Plastics are strong and waterproof material.
- b)Opaque materials do not let light pass through them.
- c)Rigid materials are easy to bend
- d)Absorbent material soaks up water easily.
Correct answer is option 'C'. Can you explain this answer?
Select the INCORRECT statement.
a)
Plastics are strong and waterproof material.
b)
Opaque materials do not let light pass through them.
c)
Rigid materials are easy to bend
d)
Absorbent material soaks up water easily.
|
|
Amit Khanna answered |
Soft materials are easy to bend.
From the following list count how many liquids are there?Chair, Juice, Books, Tables, Water, Knife, Spoon, Soup, Air, Oxygen, Tea, Shoes, Bricks, Carbon dioxide- a)4
- b)6
- c)8
- d)2
Correct answer is option 'A'. Can you explain this answer?
From the following list count how many liquids are there?
Chair, Juice, Books, Tables, Water, Knife, Spoon, Soup, Air, Oxygen, Tea, Shoes, Bricks, Carbon dioxide
a)
4
b)
6
c)
8
d)
2
|
|
Rahul Verma answered |
Liquid substances given in the list are →Juice, Water, Soup and Tea.
At what temperature, water freezes.- a)1 degree celsius
- b)5 degree celsius
- c)100 degree
- d)0 degree celsius
Correct answer is option 'D'. Can you explain this answer?
At what temperature, water freezes.
a)
1 degree celsius
b)
5 degree celsius
c)
100 degree
d)
0 degree celsius
|
|
Rahul Verma answered |
Water freezes into Ice at 0 degree celsius.
Identify the figure shown below. Select the INCORRECT STATEMENT about the figure.
Select the INCORRECT STATEMENT about the figure.- a)It is hard and strong.
- b)It has a definite shape.
- c)It can flow easily.
- d)It cannot be compressed.
Correct answer is option 'C'. Can you explain this answer?
Identify the figure shown below.

Select the INCORRECT STATEMENT about the figure.
a)
It is hard and strong.
b)
It has a definite shape.
c)
It can flow easily.
d)
It cannot be compressed.
|
|
Rahul Verma answered |
Chair is solid. In solids, the molecules are tightly packed together, so it cannot flow.
Which of the following is soluble in water?- a)Common salt
- b)Chalk powder
- c)Sand
- d)Plastic
Correct answer is option 'A'. Can you explain this answer?
Which of the following is soluble in water?
a)
Common salt
b)
Chalk powder
c)
Sand
d)
Plastic
|
|
Arun Desai answered |
Common salt is soluble in water.
Which objects shown below belongs to Box A?
 A
A
 B
B- a)

- b)

- c)

- d)

Correct answer is option 'B'. Can you explain this answer?
Which objects shown below belongs to Box A?
 A
A
 B
B
 A
A B
Ba)

b)

c)

d)


|
Nclex Coaching Centre answered |
The pin in option B belongs to box A
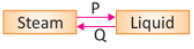 Choose the correct processes P and Q.
Choose the correct processes P and Q.- a)P-Freezing, Q- Melting
- b)P - Evaporation,Q-Condensation
- c)P-Condensation,Q- Evaporation
- d)P-Melting, Q-Freezing
Correct answer is option 'C'. Can you explain this answer?

Choose the correct processes P and Q.
a)
P-Freezing, Q- Melting
b)
P - Evaporation,Q-Condensation
c)
P-Condensation,Q- Evaporation
d)
P-Melting, Q-Freezing
|
|
Amit Khanna answered |
On condensation, gas changes into a liquid and on evaporation liquid changes into gas.
Complete the table with appropriate words Identify X, Y, Z
Identify X, Y, Z- a)Milk, Spoon, Oxygen
- b)Spoon, Milk, Oxygen
- c)Oxygen, Milk, spoon
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Complete the table with appropriate words

Identify X, Y, Z
a)
Milk, Spoon, Oxygen
b)
Spoon, Milk, Oxygen
c)
Oxygen, Milk, spoon
d)
None of the above
|
|
Amit Khanna answered |
Spoon is a solid, Milk is liquid and Oxygen is gas.
Butter paper is ______.- a)oily
- b)transparent
- c)opaque
- d)translucent
Correct answer is option 'D'. Can you explain this answer?
Butter paper is ______.
a)
oily
b)
transparent
c)
opaque
d)
translucent
|
|
Arun Desai answered |
Butter paper is translucent.
Which of the following is transparent?- a)Water
- b)Air
- c)Orange
- d)Both (a) and (b)
Correct answer is option 'D'. Can you explain this answer?
Which of the following is transparent?
a)
Water
b)
Air
c)
Orange
d)
Both (a) and (b)
|
|
Arun Desai answered |
Both air and water are transparent.
Which one of these is the hardest material?- a)Steel
- b)Butter
- c)Bread
- d)Wood
Correct answer is option 'A'. Can you explain this answer?
Which one of these is the hardest material?
a)
Steel
b)
Butter
c)
Bread
d)
Wood
|
|
Arun Desai answered |
Steel is the hardest among given materials.
Which of the following object should be crushed after use?- a)

- b)

- c)

- d)
Correct answer is option 'B'. Can you explain this answer?
Which of the following object should be crushed after use?
a)

b)

c)

d)

|
|
Swati Sharma answered |
A plastic bottle can be crushed after use.
Which of the following is a substance through which things are partially visible?- a)Opaque
- b)Translucent
- c)Transparent
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a substance through which things are partially visible?
a)
Opaque
b)
Translucent
c)
Transparent
d)
None of the above
|
|
Swati Sharma answered |
Transparent material allow partial light to pass through them.
Knife, forks and spoons are made from which material?- a)Rubber
- b)Cardboard
- c)Metal
- d)Wood
Correct answer is option 'C'. Can you explain this answer?
Knife, forks and spoons are made from which material?
a)
Rubber
b)
Cardboard
c)
Metal
d)
Wood
|
|
Arun Desai answered |
Knife, forks and spoons are made from metals.
Ice cream is the ______ state of milk.- a)Gaseous
- b)Liquid
- c)Solid
- d)All of these
Correct answer is option 'C'. Can you explain this answer?
Ice cream is the ______ state of milk.
a)
Gaseous
b)
Liquid
c)
Solid
d)
All of these
|
|
Rahul Verma answered |
Milk freezes to form Ice cream, so Ice cream is a solid state of matter.
Study the given flow chart carefully. Which of the following could be classified under groups X and Y?
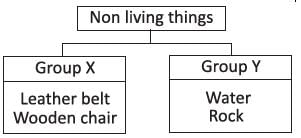
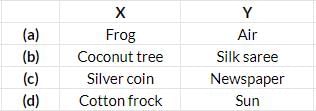
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
Study the given flow chart carefully. Which of the following could be classified under groups X and Y?



a)
a
b)
b
c)
c
d)
d
|
|
Arun Desai answered |
Cotton frock is manmade and Sun is natural.
Bicycle tires are made from which material?- a)Metal
- b)Rubber
- c)Cotton
- d)String
Correct answer is option 'B'. Can you explain this answer?
Bicycle tires are made from which material?
a)
Metal
b)
Rubber
c)
Cotton
d)
String
|
|
Arun Desai answered |
Bicycle tires are made from rubber.
Select the transparent material from the following–- a)

- b)

- c)

- d)

Correct answer is option 'D'. Can you explain this answer?
Select the transparent material from the following–
a)

b)

c)

d)

|
|
Rahul Verma answered |
Light can pass through a clean glass, so it is a transparent material.
The boiling point and freezing point of water is at- a)10°C, 100°C
- b)100°C, 0°C
- c)36.7°C, 0°C
- d)100°C, 26°C
Correct answer is option 'B'. Can you explain this answer?
The boiling point and freezing point of water is at
a)
10°C, 100°C
b)
100°C, 0°C
c)
36.7°C, 0°C
d)
100°C, 26°C
|
|
Rahul Verma answered |
The boiling point of water is 100°C and the freezing point of water is 0°C.
Which among the following is invisible?- a)Solid
- b)Liquid
- c)Gas
- d)All of these
Correct answer is option 'C'. Can you explain this answer?
Which among the following is invisible?
a)
Solid
b)
Liquid
c)
Gas
d)
All of these
|
|
Rahul Verma answered |
The Invisible form of state of matter is gaseous state.
______ is the shortest and ______ is the longest item in the given figure.


- a)Eraser, marker
- b)Pen, marker
- c)Pen, easer
- d)Eraser, pen
Correct answer is option 'D'. Can you explain this answer?
______ is the shortest and ______ is the longest item in the given figure.



a)
Eraser, marker
b)
Pen, marker
c)
Pen, easer
d)
Eraser, pen
|
|
Arun Desai answered |
Here eraser is the shortest and pen is the longest
Study the given table carefully. Which of the following can be placed at P and Q?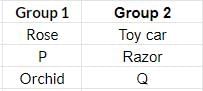
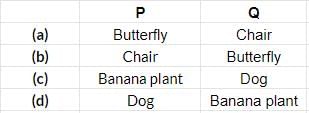
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'A'. Can you explain this answer?
Study the given table carefully. Which of the following can be placed at P and Q?


a)
a
b)
b
c)
c
d)
d
|
|
Swati Sharma answered |
Butterfly is living while chair is non-living.
Chapter doubts & questions for Materials Can Change - Science for Grade 2 2025 is part of Grade 2 exam preparation. The chapters have been prepared according to the Grade 2 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 2 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Materials Can Change - Science for Grade 2 in English & Hindi are available as part of Grade 2 exam.
Download more important topics, notes, lectures and mock test series for Grade 2 Exam by signing up for free.
Science for Grade 2
28 videos|93 docs|53 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup


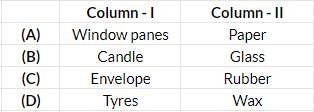
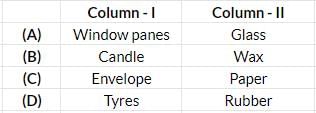




 Tissue
Tissue Paper cup
Paper cup Glass
Glass Milk carton
Milk carton








