All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Principles of Mixture Separation and Chemical Substance Purification for JAMB Exam
The formation of water from oxygen and hydrogen is a _______ .- a)Physical change
- b)Chemical change
- c)Reversible change
- d)Both Physical and Reversible change
Correct answer is option 'B'. Can you explain this answer?
The formation of water from oxygen and hydrogen is a _______ .
a)
Physical change
b)
Chemical change
c)
Reversible change
d)
Both Physical and Reversible change
|
|
Jaideep Sharma answered |
Chemical Change:
When hydrogen and oxygen combine to form water, a new substance with different properties is formed. This is a chemical change.
Explanation:
Chemical change is a process in which one or more substances are changed into new substances with different physical and chemical properties. In the formation of water from hydrogen and oxygen, the hydrogen and oxygen molecules bond together to form a new molecule of water. This reaction is accompanied by the release of energy in the form of heat and light.
The chemical equation for the reaction is:
2H2 + O2 → 2H2O
Here, two molecules of hydrogen (H2) and one molecule of oxygen (O2) combine to form two molecules of water (H2O).
Reversible Change:
The formation of water from hydrogen and oxygen is not a reversible change. Once the reaction takes place, it is not possible to separate the water molecules back into hydrogen and oxygen.
Conclusion:
Hence, the correct option is B. Chemical change.
When hydrogen and oxygen combine to form water, a new substance with different properties is formed. This is a chemical change.
Explanation:
Chemical change is a process in which one or more substances are changed into new substances with different physical and chemical properties. In the formation of water from hydrogen and oxygen, the hydrogen and oxygen molecules bond together to form a new molecule of water. This reaction is accompanied by the release of energy in the form of heat and light.
The chemical equation for the reaction is:
2H2 + O2 → 2H2O
Here, two molecules of hydrogen (H2) and one molecule of oxygen (O2) combine to form two molecules of water (H2O).
Reversible Change:
The formation of water from hydrogen and oxygen is not a reversible change. Once the reaction takes place, it is not possible to separate the water molecules back into hydrogen and oxygen.
Conclusion:
Hence, the correct option is B. Chemical change.
A coffee filter is used to separate coffee liquid from ground, this is a suitable example for- a)sublimation
- b)distillation
- c)filtration
- d)evaporation
Correct answer is option 'C'. Can you explain this answer?
A coffee filter is used to separate coffee liquid from ground, this is a suitable example for
a)
sublimation
b)
distillation
c)
filtration
d)
evaporation
|
|
Hansa Sharma answered |
Filtration is any of various mechanical, physical or biological operations that separate solids from fluids (liquids or gases) by adding a medium through which only the fluid can pass. The fluid that passes through is called the filtrate
Which of the following is not a pure substance?- a)Mercury
- b)Distilled water
- c)Nitric acid
- d)Tap water
Correct answer is 'D'. Can you explain this answer?
Which of the following is not a pure substance?
a)
Mercury
b)
Distilled water
c)
Nitric acid
d)
Tap water

|
Varun Kumar answered |
Tap water is not a pure substance because it is mixed with chemicals that purify it and if it was from the ground it has naturally occurring minerals mixed in it.
The separation of two immiscible liquids by a separating funnel depends upon:- a)The difference in their densities.
- b)The difference in their colours.
- c)The difference in their melting points.
- d)The difference in their boiling points
Correct answer is option 'A'. Can you explain this answer?
The separation of two immiscible liquids by a separating funnel depends upon:
a)
The difference in their densities.
b)
The difference in their colours.
c)
The difference in their melting points.
d)
The difference in their boiling points
|
|
Vikas Kapoor answered |
Separation by a separating funnel :
A mixture of two immiscible liquids can be separated by using a separating funnel. A separating funnel is a special type of funnel which has a stop-cock in its stem to allow the flow of a liquid from it, or to stop the flow of liquid from it. The separation of two immiscible liquids by a separating funnel depends on the difference in their densities.
A mixture of two immiscible liquids can be separated by using a separating funnel. A separating funnel is a special type of funnel which has a stop-cock in its stem to allow the flow of a liquid from it, or to stop the flow of liquid from it. The separation of two immiscible liquids by a separating funnel depends on the difference in their densities.
What type of mixture is obtained on continuous stirring when we add one spoon of sugar to water?- a)Homogeneous mixture
- b)Colloid
- c)Suspension
- d)Heterogeneous mixture
Correct answer is option 'A'. Can you explain this answer?
What type of mixture is obtained on continuous stirring when we add one spoon of sugar to water?
a)
Homogeneous mixture
b)
Colloid
c)
Suspension
d)
Heterogeneous mixture
|
|
Anita Menon answered |
The homogeneous mixture is obtained because the particles of sugar are so small that it can be diffused to the intermolecular space. The composition remains is the same throughout.
What is the basic principle behind simple distillation process?- a)Sufficient difference in the boiling points of two miscible liquids and the two liquids should boil without decomposition.
- b)The two liquids should be immiscible.
- c)Difference in boiling and melting points of two miscible liquids should be less than 30°C.
- d)The two liquids should have molecular weight greater than 200 g/mol.
Correct answer is option 'A'. Can you explain this answer?
What is the basic principle behind simple distillation process?
a)
Sufficient difference in the boiling points of two miscible liquids and the two liquids should boil without decomposition.
b)
The two liquids should be immiscible.
c)
Difference in boiling and melting points of two miscible liquids should be less than 30°C.
d)
The two liquids should have molecular weight greater than 200 g/mol.
|
|
Hansa Sharma answered |
Fractional distillation is the method used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
Two conditions essential for using this method are:
1) Two liquids must be miscible that is they totally mix with each other.
2) The difference between the boiling points of the liquids should be greater than 25K.
Which of the following colloid is a gel?- a)Fog
- b)Cheese
- c)Milk
- d)Smoke
Correct answer is option 'B'. Can you explain this answer?
Which of the following colloid is a gel?
a)
Fog
b)
Cheese
c)
Milk
d)
Smoke
|
|
Vivek Rana answered |
The colloidal system constituting the liquid as the dispersed phase and the solid as the dispersion medium is known as gel. There are some sols that have a high concentration of dispersed solid and change spontaneously into semi solid form on cooling. These are known as gels and the process is known as gelatin
The components of crude petroleum gets separated at different heights depending upon their _______ in a fractional distillation column.- a)melting points
- b)density
- c)boiling points
- d)colour
Correct answer is option 'C'. Can you explain this answer?
The components of crude petroleum gets separated at different heights depending upon their _______ in a fractional distillation column.
a)
melting points
b)
density
c)
boiling points
d)
colour
|
|
Hansa Sharma answered |
Fractional distillation is a process to separate the different components in a mixture based on the different boiling points of the components.
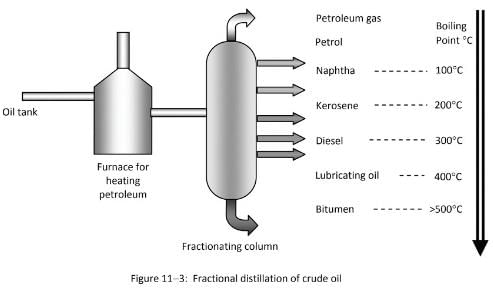
Inside the furnace, the oil is heated to about 400ºC, at which it vaporizes and passes up the fractionating column. The components or fractions condense and exit the column at different heights depending on their boiling points.

Inside the furnace, the oil is heated to about 400ºC, at which it vaporizes and passes up the fractionating column. The components or fractions condense and exit the column at different heights depending on their boiling points.
Which one of the following scrap metals cannot be separated by magnetic separation?- a)Steel
- b)Nickel
- c)Cobalt
- d)Chromium
Correct answer is option 'D'. Can you explain this answer?
Which one of the following scrap metals cannot be separated by magnetic separation?
a)
Steel
b)
Nickel
c)
Cobalt
d)
Chromium
|
|
Ravi Verma answered |
Chromium does not have magnetic properties so it cannot be separated by magnetic separation.
How can we separate a mixture of sulphur and sugar?- a)By the process of filtration
- b)By the process of crystallisation
- c)By dissolving in carbon disulphide solution
- d)By evaporation
Correct answer is option 'C'. Can you explain this answer?
How can we separate a mixture of sulphur and sugar?
a)
By the process of filtration
b)
By the process of crystallisation
c)
By dissolving in carbon disulphide solution
d)
By evaporation

|
Arka Dey answered |
Distillation is a way of separating substances based on the difference in their boiling points. Using distillation it is possible to separate sugar, which has a much higher boiling point than water, from water.
What is the correct order of the below mentioned units in water purification system?
1) Coagulation and Flocculation
2) Disinfection
3) Sedimentation tank
4) Filtration tan
- a)3, 2, 4, 1
- b)1, 2, 3, 4
- c)1, 3, 4, 2
- d)4, 3, 2, 1
Correct answer is option 'C'. Can you explain this answer?
What is the correct order of the below mentioned units in water purification system?
1) Coagulation and Flocculation
2) Disinfection
3) Sedimentation tank
4) Filtration tan
2) Disinfection
3) Sedimentation tank
4) Filtration tan
a)
3, 2, 4, 1
b)
1, 2, 3, 4
c)
1, 3, 4, 2
d)
4, 3, 2, 1
|
|
Krishna Iyer answered |
Stages of water treatment:
(i) Collection
(ii) Screening & Straining
(iii) Chemical Addition
(iv) Coagulation & Flocculation
(v) Sedimentation & Clarification
(vi) Filtration
(vii) Disinfection
(viii) Storage
(ix) Finally Distribution
Therefore, option (c) is the correct answer.
(i) Collection
(ii) Screening & Straining
(iii) Chemical Addition
(iv) Coagulation & Flocculation
(v) Sedimentation & Clarification
(vi) Filtration
(vii) Disinfection
(viii) Storage
(ix) Finally Distribution
Therefore, option (c) is the correct answer.
Which of the following involves physical change?- a)Grinding
- b)Tearing
- c)Cutting
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
Which of the following involves physical change?
a)
Grinding
b)
Tearing
c)
Cutting
d)
All of the above

|
Aryan answered |
Grinding Clearing and cutting all come under physical change..........................
If a solution contains 60g of common salt in 340g of water, the mass by mass percentage will be:- a)25 %
- b)15 %
- c)20 %
- d)17.6 %
Correct answer is 'B'. Can you explain this answer?
If a solution contains 60g of common salt in 340g of water, the mass by mass percentage will be:
a)
25 %
b)
15 %
c)
20 %
d)
17.6 %
|
|
Vivek Rana answered |
Concentration of solution is mass of solute upon mass of solution by 100 so mass of solute is 60g and mass of solvent is 340 so mass of solution is 340+60 is equal to 400 we put these values in formula so 60/400×100=15%.
A colloid with a solid dispersed phase and liquid dispersing medium is called:- a)Foam
- b)Gel
- c)Sol
- d)Emulsion
Correct answer is option 'C'. Can you explain this answer?
A colloid with a solid dispersed phase and liquid dispersing medium is called:
a)
Foam
b)
Gel
c)
Sol
d)
Emulsion

|
Vivek Kumar answered |
Because sol is a colloids in which tiny solid particles are dispersed in a liquid medium.thats why correct answer is sol
Which method is used to separate cream from milk?- a)Centrifugation
- b)Adsorption
- c)Distillation
- d)Crystallization
Correct answer is option 'A'. Can you explain this answer?
Which method is used to separate cream from milk?
a)
Centrifugation
b)
Adsorption
c)
Distillation
d)
Crystallization

|
Mayank Mehta answered |
Milk is a suspension of tiny droplets of oil (cream) in a watery liquid. The process of centrifugation is used to separate cream from milk. The milk is put in a close container in big centrifuge machine. When the centrifuge machine is switched on, the milk is rotated (or spun) at a very high speed in its container. The centrifugal force acts on the milk and due to this, the milk separates into cream and skimmed milk. The cream, being lighter, floats over the skimmed milk and can then be removed.
Name the solvent which is known as universal solvent.- a)Ethanol
- b)Benzene
- c)Vinegar
- d)Water
Correct answer is option 'D'. Can you explain this answer?
Name the solvent which is known as universal solvent.
a)
Ethanol
b)
Benzene
c)
Vinegar
d)
Water
|
|
Anita Menon answered |
Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. And, water is called the "universal solvent" because it dissolves more substances than any other liquid. This is important to every living thing on earth. It means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients.
It is water's chemical composition and physical attributes that make it such an excellent solvent. Water molecules have a polar arrangement of the oxygen and hydrogen atoms—one side (hydrogen) has a positive electrical charge and the other side (oxygen) had a negative charge. This allows the water molecule to become attracted to many other different types of molecules. Water can become so heavily attracted to a different molecule, like salt (NaCl), that it can disrupt the attractive forces that hold the sodium and chloride in the salt molecule together and, thus, dissolve it.
During filtration, the solid that remains on the filter paper is called:- a)Solute
- b)Residue
- c)Solvent
- d)Filtrate
Correct answer is option 'B'. Can you explain this answer?
During filtration, the solid that remains on the filter paper is called:
a)
Solute
b)
Residue
c)
Solvent
d)
Filtrate

|
Chinu answered |
Residue means the small amount of something remains after the main substance had gone. Thus in filtration also the solid remain is called residue.
100 mL solution contains 3.5 g salt. The mass by volume percentage (m/v) of the solution is- a)3.5 %
- b)2.5 %
- c)35 %
- d)25 %
Correct answer is option 'A'. Can you explain this answer?
100 mL solution contains 3.5 g salt. The mass by volume percentage (m/v) of the solution is
a)
3.5 %
b)
2.5 %
c)
35 %
d)
25 %
|
|
Amit Sharma answered |
FORMULA FOR MASS BY VOLUME PERCENTAGE IS = TOTAL MASS OF SOLUTE / TOTAL VOLUME × 100 MASS OF SOLUTE = 3.5 G VOLUME = 100 THERE FOR 3.5G/100 ×100= 3.5 %
Most paints are:- a)Gels
- b)Suspensions
- c)Emulsions
- d)Sols
Correct answer is option 'D'. Can you explain this answer?
Most paints are:
a)
Gels
b)
Suspensions
c)
Emulsions
d)
Sols
|
|
Naina Sharma answered |
Most paints are sols in which tiny solid particles are dispersed in a liquid medium.
Which of the following substances will not dissolve in water?- a)Sugar
- b)Sodium chloride
- c)Copper sulphate
- d)Carbon
Correct answer is option 'D'. Can you explain this answer?
Which of the following substances will not dissolve in water?
a)
Sugar
b)
Sodium chloride
c)
Copper sulphate
d)
Carbon
|
|
Amit Sharma answered |
A molecule of carbon dioxide has a slight negative charge near the oxygen and a slight positive charge near the carbon. CO2 is soluble because water molecules are attracted to these polar areas. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water.
Which one of the following statement is incorrect regarding different separation techniques?- a)Chromatography is used to separate dyes and pigments.
- b)Evaporation is used to separate components of chlorophyll.
- c)Churning technique is used for separating cream from milk.
- d)The process of evaporation is used on a large scale to obtain common salt from sea-water.
Correct answer is option 'B'. Can you explain this answer?
Which one of the following statement is incorrect regarding different separation techniques?
a)
Chromatography is used to separate dyes and pigments.
b)
Evaporation is used to separate components of chlorophyll.
c)
Churning technique is used for separating cream from milk.
d)
The process of evaporation is used on a large scale to obtain common salt from sea-water.
|
|
Hansa Sharma answered |
Evaporation is used to separate salt from seawater.
In fractional distillation, what is the purpose of the beads packed in column?- a)More liquid is distilled when beads are used in the column.
- b)To restrict the path of moving vapours.
- c)Beads provide surface for vapours to cool and condense repeatedly.
- d)Beads provide larger surface for heating.
Correct answer is option 'C'. Can you explain this answer?
In fractional distillation, what is the purpose of the beads packed in column?
a)
More liquid is distilled when beads are used in the column.
b)
To restrict the path of moving vapours.
c)
Beads provide surface for vapours to cool and condense repeatedly.
d)
Beads provide larger surface for heating.
|
|
Anita Menon answered |
The fractionating column is used to separate the liquids according to the order of their vaporisation so that they get separated as they are vaporised. The distillation process with fractional column is called fractional distillation and it is a special type of distillation in which two miscible liquids having two different boiling points but close to each other are separated by fractionating column .
Which of the following apparatus is not required in sublimation?- a)Condenser
- b)Funnel
- c)China dish
- d)Wire gauze
Correct answer is option 'A'. Can you explain this answer?
Which of the following apparatus is not required in sublimation?
a)
Condenser
b)
Funnel
c)
China dish
d)
Wire gauze
|
|
Jyoti Kapoor answered |
Condenser is a heat exchanging device in which substances condense. They lose heat and and turn into liquid from gaseous state.
An example of a colloid is:- a)Sugar solution
- b)Milk
- c)Oxygen
- d)Water
Correct answer is option 'B'. Can you explain this answer?
An example of a colloid is:
a)
Sugar solution
b)
Milk
c)
Oxygen
d)
Water
|
|
Anita Menon answered |
Types of colloids. Colloids are common in everyday life. Some examples include whipped cream, mayonnaise, milk, butter, gelatin, jelly, muddy water, plaster, colored glass, and paper. Every colloid consists of two parts: colloidal particles and the dispersing medium.
Why do we separate ethyl alcohol and water by fractional distillation?
a) The difference in the boiling point of the liquids is less than 30°C.b) Because water is a universal solvent.c) Because both of them are liquids.d) All of the aboveCorrect answer is option 'D'. Can you explain this answer?
|
|
Mayank Raj answered |
Correct answer should be, c.
Because options no.,b.is that water is not
an universal solvent.
Options A, boiling point difference should be 30kelvin
Because options no.,b.is that water is not
an universal solvent.
Options A, boiling point difference should be 30kelvin
What precaution should be taken while separating the coloured components of ink?- a)Ink should not be heated directly.
- b)Ink should be heated directly on low temperature.
- c)Ink should be heated directly on high temperature.
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
What precaution should be taken while separating the coloured components of ink?
a)
Ink should not be heated directly.
b)
Ink should be heated directly on low temperature.
c)
Ink should be heated directly on high temperature.
d)
None of the above
|
|
Ananya Sharma answered |
If ink is heated directly at high temperatures, the dye may get decomposed.
Which of the following statement is true for colloids?- a)Colloid is a homogeneous mixture.
- b)Particles of a colloid can be seen by naked eye.
- c)Particles of colloid scatter a beam of light passing through it.
- d)All of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement is true for colloids?
a)
Colloid is a homogeneous mixture.
b)
Particles of a colloid can be seen by naked eye.
c)
Particles of colloid scatter a beam of light passing through it.
d)
All of these
|
|
Vivek Rana answered |
A starch solution is a colloidal solution. in a colloidal solution, particles are relatively big. so, when a beam of light is passed, the path of light is visible. it scatters. this scattering of beam of light through a colloidal solution is called tyndall effect.
Which technique is used in diagnostic laboratories for blood and urine test?- a)Sublimation
- b)Centrifugation
- c)Evaporation
- d)Magnetic separation
Correct answer is option 'B'. Can you explain this answer?
Which technique is used in diagnostic laboratories for blood and urine test?
a)
Sublimation
b)
Centrifugation
c)
Evaporation
d)
Magnetic separation

|
Nikhil Mehra answered |
Centrifugation is a technique used in diagnostic labs for blood and urine tests.The principle of centrifugation is that denser particles are forced to the bottom and the lighter particles float on the surface.
Which of the following statements is incorrect about physical changes?- a)There is no gain or loss of energy.
- b)It is permanent and irreversible.
- c)Composition of the substance remains same.
- d)No new substance is formed.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements is incorrect about physical changes?
a)
There is no gain or loss of energy.
b)
It is permanent and irreversible.
c)
Composition of the substance remains same.
d)
No new substance is formed.
|
|
Gopal Majumdar answered |
Explanation:
Physical changes are changes in the physical properties of a substance, such as shape, size, state, or appearance. They do not involve any change in the chemical composition of the substance. Some common examples of physical changes are melting, freezing, boiling, condensation, sublimation, and dissolution.
Now, let us analyze the given statements one by one:
a) There is no gain or loss of energy: This statement is correct. Physical changes do not involve any change in the energy of the substance. The energy may be required to carry out the change, but it is not gained or lost by the substance itself.
b) It is permanent and irreversible: This statement is incorrect. Physical changes are usually temporary and reversible. For example, if we melt an ice cube, it will turn into water, but we can freeze the water again to get back the ice cube. Similarly, if we dissolve salt in water, we can evaporate the water to get back the salt.
c) Composition of the substance remains the same: This statement is correct. Physical changes do not involve any change in the chemical composition of the substance. The atoms or molecules of the substance remain the same before and after the change.
d) No new substance is formed: This statement is correct. Physical changes do not involve any chemical reaction, so no new substance is formed. The substance may change its state or appearance, but it remains the same substance.
Therefore, the correct answer is option 'B' which says that physical changes are permanent and irreversible. In reality, physical changes are usually temporary and reversible.
Physical changes are changes in the physical properties of a substance, such as shape, size, state, or appearance. They do not involve any change in the chemical composition of the substance. Some common examples of physical changes are melting, freezing, boiling, condensation, sublimation, and dissolution.
Now, let us analyze the given statements one by one:
a) There is no gain or loss of energy: This statement is correct. Physical changes do not involve any change in the energy of the substance. The energy may be required to carry out the change, but it is not gained or lost by the substance itself.
b) It is permanent and irreversible: This statement is incorrect. Physical changes are usually temporary and reversible. For example, if we melt an ice cube, it will turn into water, but we can freeze the water again to get back the ice cube. Similarly, if we dissolve salt in water, we can evaporate the water to get back the salt.
c) Composition of the substance remains the same: This statement is correct. Physical changes do not involve any change in the chemical composition of the substance. The atoms or molecules of the substance remain the same before and after the change.
d) No new substance is formed: This statement is correct. Physical changes do not involve any chemical reaction, so no new substance is formed. The substance may change its state or appearance, but it remains the same substance.
Therefore, the correct answer is option 'B' which says that physical changes are permanent and irreversible. In reality, physical changes are usually temporary and reversible.
What do you understand by the term concentrated solution?- a)Solution containing no solute
- b)Solution with low solute concentration
- c)Solution in which no more solute can be dissolved
- d)Solution with high solute concentration
Correct answer is option 'D'. Can you explain this answer?
What do you understand by the term concentrated solution?
a)
Solution containing no solute
b)
Solution with low solute concentration
c)
Solution in which no more solute can be dissolved
d)
Solution with high solute concentration
|
|
Sarita Reddy answered |
A concentrated solution contains a relatively large amount of the solute in the same volume of solvent. Most commercial acids are concentrated solutions. For example, commercial hydrochloric acid (HCl) and sulfuric acid (H2SO4) are concentrated solutions.
A dilute solution is one that contains a relatively small amount of the solute in a given volume of solvent. Tap water is an example of a dilute
solution; it contains very small quantities of dissolved minerals.
Which technique is used to separate drugs from blood?- a)Sublimation
- b)Chromatography
- c)Centrifugation
- d)Evaporation
Correct answer is option 'B'. Can you explain this answer?
Which technique is used to separate drugs from blood?
a)
Sublimation
b)
Chromatography
c)
Centrifugation
d)
Evaporation
|
|
Naina rao answered |
Chromatography is the method used to separate drugs from the blood. Chromatography is a technique used to separate components in a mixture on the basis of them being mixed as mobile phase i.e. liquid or gas and stationary phase i.e. solid or liquid.
Which of the following is not true for a mixture?- a)A mixture can be separated into its constituents.
- b)Energy is not given out in the preparation of a mixture.
- c)A mixture has a fixed melting point.
- d)The composition of a mixture is variable
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not true for a mixture?
a)
A mixture can be separated into its constituents.
b)
Energy is not given out in the preparation of a mixture.
c)
A mixture has a fixed melting point.
d)
The composition of a mixture is variable
|
|
Jyoti Kapoor answered |
A mixture does not have a fixed melting point, boiling point etc. On the other hand, compounds have a a fixed melting point, boiling point.
Which of the following statement is correct?- a)Both burning of a paper and cooking of food are examples of a physical change
- b)Both burning of wood and cooking of food are are examples of a chemical change.
- c)Burning of a paper is a physical change and cooking of food is a chemical change.
- d)Burning of a paper is chemical change and cooking of food is a physical change.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statement is correct?
a)
Both burning of a paper and cooking of food are examples of a physical change
b)
Both burning of wood and cooking of food are are examples of a chemical change.
c)
Burning of a paper is a physical change and cooking of food is a chemical change.
d)
Burning of a paper is chemical change and cooking of food is a physical change.
|
|
Avinash Patel answered |
Examples of Chemical Changes
A new compound (product) results from a chemical change as the atoms rearrange themselves to form new chemical bonds.
A new compound (product) results from a chemical change as the atoms rearrange themselves to form new chemical bonds.
- Burning wood
- Souring milk
- Mixing acid and base
- Digesting food
- Cooking an egg
- Heating sugar to form caramel
- Baking a cake
- Rusting of iron
Which of the following statement is not true?- a)The solubility of gases in liquids decreases on increasing the temperature.
- b)The solubility of solids in liquids remain unaffected by changes in pressure.
- c)The solubility of solids in liquids increases on increasing the temperature.
- d)The solubility of gases in liquids increases on increasing the temperature.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is not true?
a)
The solubility of gases in liquids decreases on increasing the temperature.
b)
The solubility of solids in liquids remain unaffected by changes in pressure.
c)
The solubility of solids in liquids increases on increasing the temperature.
d)
The solubility of gases in liquids increases on increasing the temperature.
|
|
Jyoti Kapoor answered |
The reason for this gas solubility relationship with temperature is very similar to the reason that vapor pressure increases with temperature. Increased temperature causes an increase in kinetic energy. The higher kinetic energy causes more motion in molecules which break intermolecular bonds and escape from solution. As the temperature increases, the solubility of a gas decreases.
Which technique is used to separate blood cells from plasma?- a)Evaporation
- b)Sublimation
- c)Centrifugation
- d)Filtration
Correct answer is option 'C'. Can you explain this answer?
Which technique is used to separate blood cells from plasma?
a)
Evaporation
b)
Sublimation
c)
Centrifugation
d)
Filtration

|
Vandana Rane answered |
Blood separation is one of the crucial processes in a clinical lab and is usually conducted via a process called centrifugation.
The clear liquid which is left behind in the beaker after settling down of the sediments is called:- a)Solvent
- b)Supernatant liquid
- c)Solution
- d)Sediment
Correct answer is option 'B'. Can you explain this answer?
The clear liquid which is left behind in the beaker after settling down of the sediments is called:
a)
Solvent
b)
Supernatant liquid
c)
Solution
d)
Sediment

|
Ishita Chakraborty answered |
Sedimantation and decantation method are used to separate a mixture containing an insoluble solid in a liquid. In this method, the mixture is allowed to stand undisturbed for sometime. The insoluble solid substance settles down and a clear liquid is left standing. This clear liquid is called as supernatant liquid. The solid substance that settles down is called sediment. This entire process is known as sedimentation.
The clear water (supernatant liquid) is then poured out carefully into another beaker, leaving the sediments undisturbed. This process is known as decantation.
Burning of a candle is an example of:- a)Physical change
- b)Physical and chemical change
- c)Chemical change
- d)Reversible change
Correct answer is option 'B'. Can you explain this answer?
Burning of a candle is an example of:
a)
Physical change
b)
Physical and chemical change
c)
Chemical change
d)
Reversible change
|
|
Ravi Verma answered |
Burning of candle is both physical and chemical change. Burning of candle melts the wax and hence physical state of wax has changed from solid to liquid. Again the wax combines with the atmosphere oxygen and changes to carbon dioxide, heat and light.
Thus both the changes are accompanied in the burning of candle.
Which one of the following laboratory apparatus is not used in the process of evaporation?- a)Wire gauze
- b)Burner
- c)Funnel
- d)Evaporating dish
Correct answer is option 'C'. Can you explain this answer?
Which one of the following laboratory apparatus is not used in the process of evaporation?
a)
Wire gauze
b)
Burner
c)
Funnel
d)
Evaporating dish

|
Aryan answered |
Funnel because in funnel there is no use of heat and evaporation cause by heating and other are resistant to heating while funnel is not registered to heating.
Which of the following method can be used to separate a mixture of camphor and sugar?- a)Sublimation
- b)Filtration
- c)Distillation
- d)Crystallisation
Correct answer is option 'A'. Can you explain this answer?
Which of the following method can be used to separate a mixture of camphor and sugar?
a)
Sublimation
b)
Filtration
c)
Distillation
d)
Crystallisation
|
|
Arun Sharma answered |
Sublimation is the process which is used to separate the mixture of camphor and salt. Camphor gets directly converted to its gaseous state, leaving the salt behind.
A compound is a _______ substance made up of _______ .- a)impure; two or more simpler substances
- b)soft; only one kind of atoms
- c)hard; only one element
- d)pure; two or more elements
Correct answer is option 'D'. Can you explain this answer?
A compound is a _______ substance made up of _______ .
a)
impure; two or more simpler substances
b)
soft; only one kind of atoms
c)
hard; only one element
d)
pure; two or more elements
|
|
Ananya Sharma answered |
A molecule is the smallest particle of a substance that exists independently. Molecules of most elements are made up of only one of atom of that element. Oxygen, along with nitrogen, hydrogen, and chlorine are made up of two atoms.
Which of the following is an example of a pure substance?- a)Air
- b)Brass
- c)Water
- d)Milk
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of a pure substance?
a)
Air
b)
Brass
c)
Water
d)
Milk
|
|
Priyanka Barat answered |
Air is a mixture of gases (mainly nitrogen and oxygen, plus others).
Brass is an alloy (a mixture of copper and zinc).
Milk is a colloid (a mixture of water, fats, proteins, etc.).
Water (H₂O) is a pure substance because it has a fixed chemical composition.
The answer is Water (C) because it’s made of only one kind of molecule, H₂O. The others are mixtures: air is gases, brass is metals, and milk is a colloid. That’s why water is the only pure substance here.
Brass is an alloy (a mixture of copper and zinc).
Milk is a colloid (a mixture of water, fats, proteins, etc.).
Water (H₂O) is a pure substance because it has a fixed chemical composition.
The answer is Water (C) because it’s made of only one kind of molecule, H₂O. The others are mixtures: air is gases, brass is metals, and milk is a colloid. That’s why water is the only pure substance here.
Freezing, condensation and evaporation processes are examples of:- a)Chemical change
- b)Physical change
- c)Both physical and chemical change
- d)Neither physical change nor chemical change
Correct answer is option 'B'. Can you explain this answer?
Freezing, condensation and evaporation processes are examples of:
a)
Chemical change
b)
Physical change
c)
Both physical and chemical change
d)
Neither physical change nor chemical change

|
Yajush Srivastava answered |
The correct answer is option B. This is because the process of freezing , condensation and evaporation are the processes by which the state of any substance is changed. So as we all know that the process of state change is always a physical process , so freezing , evaporation and condensation are the examples of the Physical Changes.
.
.
.
If u thought my answer was useful then please upvote my answer
.
.
I hope it was useful
.
.
.
If u thought my answer was useful then please upvote my answer
.
.
I hope it was useful
Which of the following parameters of a substance does not alter during a physical change?- a)State
- b)Mass
- c)Volume
- d)Size
Correct answer is option 'B'. Can you explain this answer?
Which of the following parameters of a substance does not alter during a physical change?
a)
State
b)
Mass
c)
Volume
d)
Size
|
|
Hansa Sharma answered |
A physical change can affect the size, shape or color of a substance but does not affect its composition or mass. The substances may be changed to another phase (i.e. gas, liquid, solid) or separated or combined.
Examples:
when ice melts
when sulfur is mixed with iron filings.
breaking a glass
dissolving sugar in water
when sulfur is mixed with iron filings.
breaking a glass
dissolving sugar in water
What is the use of sedimentation tank in water purification system?- a)To separate out the insoluble substances from water.
- b)To separate out very small suspended particles from water.
- c)To kill the germs present in water.
- d)All of the above.
Correct answer is option 'A'. Can you explain this answer?
What is the use of sedimentation tank in water purification system?
a)
To separate out the insoluble substances from water.
b)
To separate out very small suspended particles from water.
c)
To kill the germs present in water.
d)
All of the above.
|
|
Ananya Das answered |
- In water, some particles are suspended and to remove these particles sedimentation tank is used.
- It is based on the gravity, some particles in suspended waste have a gravity similar to water so they remain suspended.
- But some waste particles are heavier due to their heavier weight, they get settle down in the bottom so sedimentation tank remove it very easily.
- The particles which get settle down on the tank we called that sludge. so we can easily remove this sludge with the help of sedimentation tank.
Which solvent can be used to obtain sodium chloride from a mixture of sodium chloride and sulphur without using water?- a)Sodium hydroxide
- b)Carbon disulphide
- c)Sulphuric acid
- d)Kerosene
Correct answer is option 'B'. Can you explain this answer?
Which solvent can be used to obtain sodium chloride from a mixture of sodium chloride and sulphur without using water?
a)
Sodium hydroxide
b)
Carbon disulphide
c)
Sulphuric acid
d)
Kerosene
|
|
Sarita Reddy answered |
Carbon disulphide is a suitable solvent for sulphur. Sodium chloride remains undissolved and can be separated by filtration process.
What will you observe when a mixture of iodine and salt is heated in a china dish?- a)No change in the china dish is observed.
- b)Salt is left behind in the china dish.
- c)Iodine is left behind in the china dish.
- d)The mixture starts melting
Correct answer is option 'B'. Can you explain this answer?
What will you observe when a mixture of iodine and salt is heated in a china dish?
a)
No change in the china dish is observed.
b)
Salt is left behind in the china dish.
c)
Iodine is left behind in the china dish.
d)
The mixture starts melting
|
|
Avinash Patel answered |
Iodine sublimes on heating and salt will be left behind in the china dish.
Which of the following method is used to separate a mixture of iron nails and sodium chloride?- a)Separating funnel
- b)Filtration
- c)Magnetic separation
- d)Distillation
Correct answer is option 'C'. Can you explain this answer?
Which of the following method is used to separate a mixture of iron nails and sodium chloride?
a)
Separating funnel
b)
Filtration
c)
Magnetic separation
d)
Distillation
|
|
Vikram Khanna answered |
Magnetic separation is a process in which magnetically susceptible material is extracted from a mixture using a magnetic force. This separation technique can be useful in mining iron as it is attracted to a magnet.
What type of change takes place when a sodium hydroxide pellet is added to water?- a)Reversible change
- b)Reversible chemical change
- c)Physical change
- d)Chemical change
Correct answer is option 'D'. Can you explain this answer?
What type of change takes place when a sodium hydroxide pellet is added to water?
a)
Reversible change
b)
Reversible chemical change
c)
Physical change
d)
Chemical change
|
|
Vikas Kapoor answered |
Because sodium hydroxide is a type of chemical and when we add it to water than this will be a chemical activity.
What is the principle behind the process of centrifugation?- a)Particles are separated based on the difference in colour.
- b)Denser particles are forced to the bottom and lighter particles stay at the top when spun rapidly.
- c)Lighter particles are forced to the bottom and denser particles stay at the top when spun rapidly.
- d)Particles are separated based on the difference in temperature.
Correct answer is option 'B'. Can you explain this answer?
What is the principle behind the process of centrifugation?
a)
Particles are separated based on the difference in colour.
b)
Denser particles are forced to the bottom and lighter particles stay at the top when spun rapidly.
c)
Lighter particles are forced to the bottom and denser particles stay at the top when spun rapidly.
d)
Particles are separated based on the difference in temperature.

|
Jhanvi Mukherjee answered |
The centrifugation depends upon the size, shape, and density of the particles. It also depends on the viscosity of the medium and the speed of rotation. The theory is that the lighter particles stay at the top when spun rapidly, the denser particles are forced to the bottom.
Chapter doubts & questions for Principles of Mixture Separation and Chemical Substance Purification - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Principles of Mixture Separation and Chemical Substance Purification - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









