All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Aromatic Hydrocarbons- Benzene for JAMB Exam
- a)Fittig reaction
- b)Wurtz’s-Fittig reaction
- c)Ullmann reaction
- d)Wurtz’s reaction
Correct answer is option 'B'. Can you explain this answer?
a)
Fittig reaction
b)
Wurtz’s-Fittig reaction
c)
Ullmann reaction
d)
Wurtz’s reaction
|
|
Om Desai answered |
The correct answer is option B
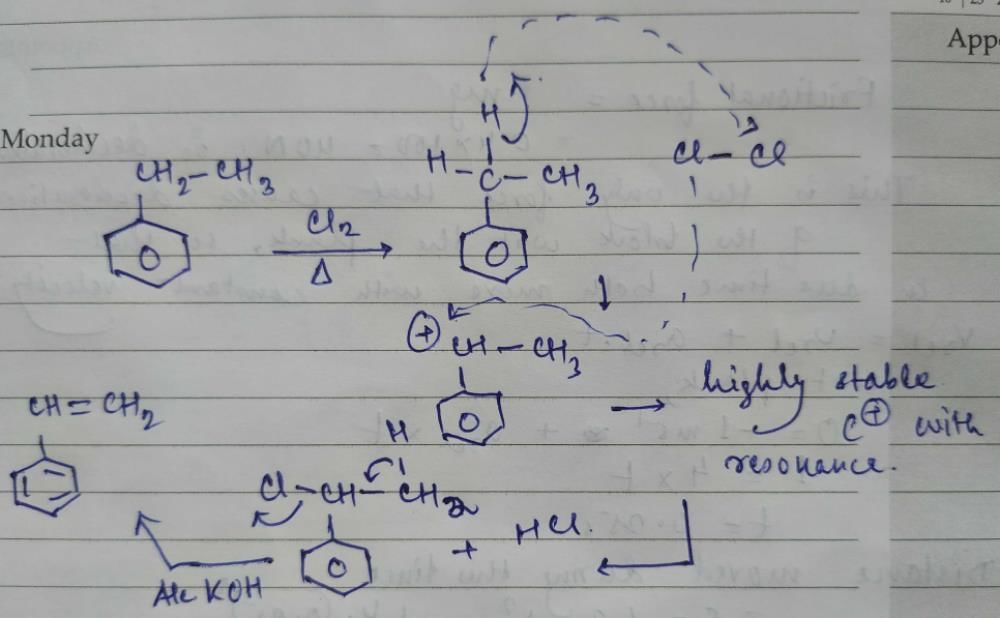
Wurtz - Fittig reaction:
Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene.
For example, bromobenzene reacts with methyl bromide in presence of sodium. dry ether to form toluene.
C6H6 - Br + CH3 - Br + 2Na(dry ether)------> C6H5 - CH3 + 2NaBr
Wurtz - Fittig reaction:
Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene.
For example, bromobenzene reacts with methyl bromide in presence of sodium. dry ether to form toluene.
C6H6 - Br + CH3 - Br + 2Na(dry ether)------> C6H5 - CH3 + 2NaBr
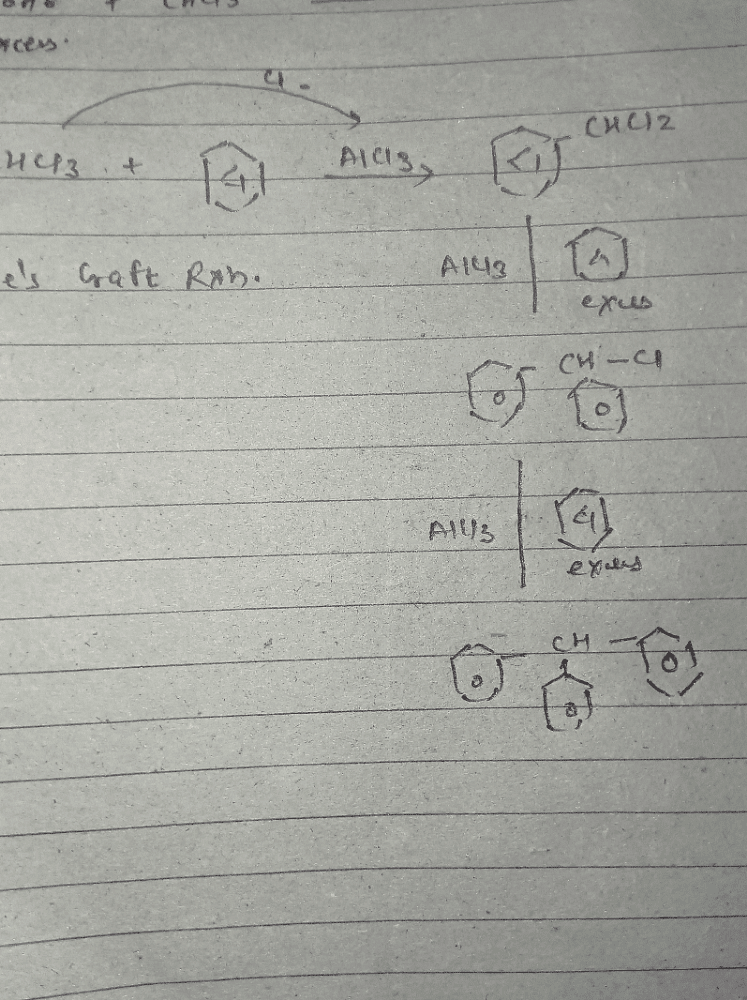
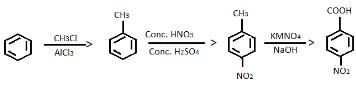
Provide the appropriate sequence of reagents that can bring about the following transformation.
- a)CH3CI/AICI3 then conc.HNO3/conc.H2SO4 then KMnO4/NaOH
- b)cone. HNO3 /conc.H2SO4 then CH3CI/AICI3 then KMnO4/NaOH
- c)CH3CI/AICI3 then KMnO4/NaOH then conc.HNO3/conc.H2SO4
- d)conc.H2SO4 /conc.HNO3 then CH3COCI/AICI3 then KMnO4/NaOH
Correct answer is option 'A'. Can you explain this answer?
Provide the appropriate sequence of reagents that can bring about the following transformation.
a)
CH3CI/AICI3 then conc.HNO3/conc.H2SO4 then KMnO4/NaOH
b)
cone. HNO3 /conc.H2SO4 then CH3CI/AICI3 then KMnO4/NaOH
c)
CH3CI/AICI3 then KMnO4/NaOH then conc.HNO3/conc.H2SO4
d)
conc.H2SO4 /conc.HNO3 then CH3COCI/AICI3 then KMnO4/NaOH
|
|
Naina Sharma answered |
The correct answer is Option A.

Which of the following is not a property of benzene?- a)It is a colorless liquid.
- b)It has a sweet odor.
- c)It is insoluble in water.
- d)It readily undergoes addition reactions.
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a property of benzene?
a)
It is a colorless liquid.
b)
It has a sweet odor.
c)
It is insoluble in water.
d)
It readily undergoes addition reactions.
|
|
Adaeze Igwe answered |
Properties of Benzene
Colorless Liquid
Benzene is a colorless liquid with a characteristic sweet odor. It is commonly used as a solvent in various industries due to its liquid form.
Sweet Odor
One of the distinctive properties of benzene is its sweet odor, which can be detected even at low concentrations. However, it is important to note that benzene exposure can be harmful and should be handled with caution.
Insoluble in Water
Benzene is insoluble in water but is soluble in organic solvents such as ether, alcohol, and acetone. This property makes it a useful solvent for various organic compounds.
Does not Readily Undergo Addition Reactions
Benzene is known for its resistance to addition reactions, unlike typical alkenes. Instead, benzene undergoes substitution reactions, where one atom or group of atoms is replaced by another. This stability is due to the delocalization of electrons in the benzene ring.
In conclusion, the property of benzene that sets it apart from typical alkenes is its resistance to addition reactions. Instead, benzene undergoes substitution reactions, making it a unique and versatile compound in organic chemistry.
Which of the following is not a general property of aromatic hydrocarbons?- a)Stability
- b)Nonpolar nature
- c)High boiling points
- d)Low melting points
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a general property of aromatic hydrocarbons?
a)
Stability
b)
Nonpolar nature
c)
High boiling points
d)
Low melting points
|
|
Hauwa Lawal answered |
Understanding Aromatic Hydrocarbons
Aromatic hydrocarbons are a class of compounds that have unique properties due to their stable ring structures and delocalized π-electrons. To understand why low melting points (option 'D') is not a general property of aromatic hydrocarbons, let’s delve into their characteristics.
1. Stability
- Aromatic compounds are exceptionally stable due to resonance. The delocalization of electrons in the π-system contributes to this stability, allowing them to withstand chemical reactions that would typically affect aliphatic compounds.
2. Nonpolar Nature
- Aromatic hydrocarbons are primarily nonpolar. This nonpolarity arises from their symmetrical structure, making them insoluble in water, while soluble in organic solvents.
3. High Boiling Points
- Aromatic hydrocarbons tend to have high boiling points compared to their aliphatic counterparts. This is due to the presence of strong π-π stacking interactions and van der Waals forces among the stacked aromatic rings.
4. Low Melting Points (Incorrect Statement)
- Contrary to the statement, many aromatic hydrocarbons exhibit relatively high melting points. This is because their planar, symmetrical structures allow for efficient packing in the solid state, leading to stronger intermolecular interactions. Compounds like naphthalene have significant melting points, highlighting this trend.
Conclusion
In summary, low melting points is not a characteristic of aromatic hydrocarbons. Instead, they often showcase relatively high melting points due to their unique structural properties and intermolecular forces. This distinction makes option 'D' the correct answer in this context.
Aromatic hydrocarbons are a class of compounds that have unique properties due to their stable ring structures and delocalized π-electrons. To understand why low melting points (option 'D') is not a general property of aromatic hydrocarbons, let’s delve into their characteristics.
1. Stability
- Aromatic compounds are exceptionally stable due to resonance. The delocalization of electrons in the π-system contributes to this stability, allowing them to withstand chemical reactions that would typically affect aliphatic compounds.
2. Nonpolar Nature
- Aromatic hydrocarbons are primarily nonpolar. This nonpolarity arises from their symmetrical structure, making them insoluble in water, while soluble in organic solvents.
3. High Boiling Points
- Aromatic hydrocarbons tend to have high boiling points compared to their aliphatic counterparts. This is due to the presence of strong π-π stacking interactions and van der Waals forces among the stacked aromatic rings.
4. Low Melting Points (Incorrect Statement)
- Contrary to the statement, many aromatic hydrocarbons exhibit relatively high melting points. This is because their planar, symmetrical structures allow for efficient packing in the solid state, leading to stronger intermolecular interactions. Compounds like naphthalene have significant melting points, highlighting this trend.
Conclusion
In summary, low melting points is not a characteristic of aromatic hydrocarbons. Instead, they often showcase relatively high melting points due to their unique structural properties and intermolecular forces. This distinction makes option 'D' the correct answer in this context.
Which of the following is commonly used as a solvent in industrial applications?- a)Acetylene
- b)Toluene
- c)Ethylene
- d)Propylene
Correct answer is option 'B'. Can you explain this answer?
Which of the following is commonly used as a solvent in industrial applications?
a)
Acetylene
b)
Toluene
c)
Ethylene
d)
Propylene
|
|
Chukwudi Eze answered |
Overview of Solvents in Industrial Applications
In industrial applications, solvents play a crucial role in processes such as extraction, purification, and chemical reactions. The choice of solvent can significantly affect the efficiency and outcome of these processes.
Toluene as a Common Solvent
Toluene is a widely used solvent in various industrial applications for several reasons:
Comparison with Other Options
- Acetylene: Primarily used as a fuel and in synthesis, but not commonly used as a solvent.
- Ethylen: Mainly utilized in polymer production, not effective as a solvent.
- Propylene: Mostly used in the production of polypropylene and other chemicals, not typically a solvent.
Conclusion
Toluene's unique properties make it a preferred choice in numerous industrial applications, solidifying its role as a vital solvent. Its effectiveness in dissolving various compounds and versatility in applications highlight why option 'B' is the correct answer.
In industrial applications, solvents play a crucial role in processes such as extraction, purification, and chemical reactions. The choice of solvent can significantly affect the efficiency and outcome of these processes.
Toluene as a Common Solvent
Toluene is a widely used solvent in various industrial applications for several reasons:
- Effective Solubility: Toluene has the ability to dissolve a wide range of organic compounds, making it ideal for processes that require the dissolution of materials.
- Low Boiling Point: With a boiling point of approximately 110°C, toluene is effective in processes that require evaporation or distillation, allowing for easy separation of compounds.
- Chemical Stability: Toluene is chemically stable under a variety of conditions, which minimizes the risk of unwanted reactions during industrial processes.
- Wide Applications: It is commonly used in paints, coatings, adhesives, and as a reagent in chemical syntheses, demonstrating its versatility as a solvent.
Comparison with Other Options
- Acetylene: Primarily used as a fuel and in synthesis, but not commonly used as a solvent.
- Ethylen: Mainly utilized in polymer production, not effective as a solvent.
- Propylene: Mostly used in the production of polypropylene and other chemicals, not typically a solvent.
Conclusion
Toluene's unique properties make it a preferred choice in numerous industrial applications, solidifying its role as a vital solvent. Its effectiveness in dissolving various compounds and versatility in applications highlight why option 'B' is the correct answer.
Passage IAn aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI Q. If all the monochloro isomers of P are considered, how many of them are possible?- a)4
- b)6
- c)7
- d)8
Correct answer is option 'D'. Can you explain this answer?
Passage I
An aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI
Q. If all the monochloro isomers of P are considered, how many of them are possible?
a)
4
b)
6
c)
7
d)
8

|
Ayush Kumar answered |
7???
Aromatic hydrocarbons are commonly found in:- a)Natural gas deposits
- b)Volcanic eruptions
- c)Ocean water
- d)Lunar rocks
Correct answer is option 'A'. Can you explain this answer?
Aromatic hydrocarbons are commonly found in:
a)
Natural gas deposits
b)
Volcanic eruptions
c)
Ocean water
d)
Lunar rocks
|
|
Deepak Iyer answered |
Aromatic hydrocarbons are often found in natural gas deposits alongside other hydrocarbon compounds. They can be extracted and processed for various industrial applications.
What is the major product of the following reaction?
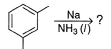
- a)
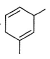
- b)
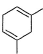
- c)
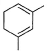
- d)
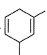
Correct answer is option 'B'. Can you explain this answer?
What is the major product of the following reaction?
a)
b)
c)
d)

|
Ciel Knowledge answered |
The reaction shown in the image is a Birch reduction. The Birch reduction is a process typically involving the reduction of aromatic hydrocarbons to cyclohexadienes. The reactants in the reaction, sodium (Na) and ammonia (NH3), suggest that this is indeed a Birch reduction.
In a Birch reduction, the aromatic ring is partially reduced, and the position of the double bonds in the resulting product is very important. The electrons from the alkali metal (in this case, sodium) are transferred to the aromatic ring, and the protons from the ammonia solvent are added to the carbon atoms of the aromatic ring.
The correct product of a Birch reduction of benzene (the starting aromatic compound in the image) would be 1,4-cyclohexadiene. The double bonds in the product are at the 1 and 4 positions of the cyclohexadiene ring, because the Birch reduction specifically avoids adding hydrogen atoms to adjacent carbon atoms.
Therefore, the major product of the reaction shown in the image would be the compound labeled as number 2, which represents 1,4-cyclohexadiene.
What is/are true regarding Friedel-Crafts methylation reaction of phenol using CH3CI/AICI3?- a)Methylation succeeds only if large excess of AICI3 is taken
- b)AICI3 reacts with phenol to give C6H5OAICI2
- c)Methylation occurs mainly at meta position
- d)Ortholpara methylation occur with para isomer as major product
Correct answer is option 'A,B,D'. Can you explain this answer?
What is/are true regarding Friedel-Crafts methylation reaction of phenol using CH3CI/AICI3?
a)
Methylation succeeds only if large excess of AICI3 is taken
b)
AICI3 reacts with phenol to give C6H5OAICI2
c)
Methylation occurs mainly at meta position
d)
Ortholpara methylation occur with para isomer as major product
|
|
Karan Karan answered |
Aromatic hydrocarbons can undergo substitution reactions due to:- a)High reactivity
- b)Delocalized pi electrons
- c)Double bonds
- d)Presence of oxygen
Correct answer is option 'B'. Can you explain this answer?
Aromatic hydrocarbons can undergo substitution reactions due to:
a)
High reactivity
b)
Delocalized pi electrons
c)
Double bonds
d)
Presence of oxygen
|
|
Adaobi Eze answered |
Delocalized Pi Electrons:
Aromatic hydrocarbons have a unique structure known as a benzene ring, which consists of alternating single and double bonds between carbon atoms. The delocalized pi electrons in the benzene ring create a stable and electron-rich system. This electron-rich system makes the aromatic hydrocarbons more susceptible to undergoing substitution reactions.
Substitution Reactions:
In a substitution reaction, a functional group or atom is replaced by another functional group or atom. The delocalized pi electrons in aromatic hydrocarbons can attract electrophiles, which are species that are electron-deficient and seek electrons to stabilize themselves. The electrophile can then undergo a substitution reaction with the aromatic hydrocarbon, resulting in the replacement of a hydrogen atom on the benzene ring.
Reactivity:
The high reactivity of aromatic hydrocarbons in substitution reactions is primarily due to the presence of delocalized pi electrons. These electrons provide a source of electron density that can interact with electrophiles, leading to the formation of new functional groups on the benzene ring.
In summary, the delocalized pi electrons in aromatic hydrocarbons play a crucial role in their reactivity towards substitution reactions. This unique electronic structure makes aromatic hydrocarbons more prone to undergoing substitution reactions compared to other hydrocarbons.
Aromatic hydrocarbons have a unique structure known as a benzene ring, which consists of alternating single and double bonds between carbon atoms. The delocalized pi electrons in the benzene ring create a stable and electron-rich system. This electron-rich system makes the aromatic hydrocarbons more susceptible to undergoing substitution reactions.
Substitution Reactions:
In a substitution reaction, a functional group or atom is replaced by another functional group or atom. The delocalized pi electrons in aromatic hydrocarbons can attract electrophiles, which are species that are electron-deficient and seek electrons to stabilize themselves. The electrophile can then undergo a substitution reaction with the aromatic hydrocarbon, resulting in the replacement of a hydrogen atom on the benzene ring.
Reactivity:
The high reactivity of aromatic hydrocarbons in substitution reactions is primarily due to the presence of delocalized pi electrons. These electrons provide a source of electron density that can interact with electrophiles, leading to the formation of new functional groups on the benzene ring.
In summary, the delocalized pi electrons in aromatic hydrocarbons play a crucial role in their reactivity towards substitution reactions. This unique electronic structure makes aromatic hydrocarbons more prone to undergoing substitution reactions compared to other hydrocarbons.
Which of the following aromatic hydrocarbons is commonly used as a fuel additive?- a)Xylene
- b)Phenol
- c)Naphthalene
- d)Aniline
Correct answer is option 'A'. Can you explain this answer?
Which of the following aromatic hydrocarbons is commonly used as a fuel additive?
a)
Xylene
b)
Phenol
c)
Naphthalene
d)
Aniline
|
|
Deepak Iyer answered |
Xylene, an aromatic hydrocarbon, is commonly used as a fuel additive to improve the octane rating of gasoline. It enhances the combustion properties of the fuel.
Benzene is classified as which type of compound?- a)Alkane
- b)Alkene
- c)Aromatic compound
- d)Alkyne
Correct answer is option 'C'. Can you explain this answer?
Benzene is classified as which type of compound?
a)
Alkane
b)
Alkene
c)
Aromatic compound
d)
Alkyne
|
|
Deepak Iyer answered |
Benzene is classified as an aromatic compound due to its cyclic structure and the presence of delocalized pi electrons. It displays unique properties and reactivity patterns distinct from those of alkanes, alkenes, and alkynes.
Benzene can be oxidized to form which of the following compounds?- a)Ethanol
- b)Acetic acid
- c)Propanol
- d)Methanol
Correct answer is option 'B'. Can you explain this answer?
Benzene can be oxidized to form which of the following compounds?
a)
Ethanol
b)
Acetic acid
c)
Propanol
d)
Methanol
|
|
Deepak Iyer answered |
Benzene can undergo oxidation to form acetic acid (CH3COOH) under suitable conditions and with the use of appropriate reagents.
Prolonged exposure to benzene is associated with which health hazard?- a)Respiratory irritation
- b)Liver damage
- c)Kidney failure
- d)Bone fractures
Correct answer is option 'B'. Can you explain this answer?
Prolonged exposure to benzene is associated with which health hazard?
a)
Respiratory irritation
b)
Liver damage
c)
Kidney failure
d)
Bone fractures
|
|
Deepak Iyer answered |
Prolonged or excessive exposure to benzene has been linked to adverse health effects, including liver damage. Benzene is a known carcinogen and can also affect bone marrow, leading to blood disorders.
What is the major product of the following reaction?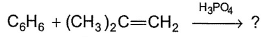
- a)
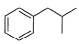
- b)
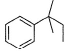
- c)
- d)Both “b” and “c”
Correct answer is option 'B'. Can you explain this answer?
What is the major product of the following reaction?
a)
b)
c)
d)
Both “b” and “c”

|
Harika Chittiboyina answered |
The mechanism underneath is fridel crafts alkylation.
The double bond breaks and partial +ve occur on the secondary carbon.
It attaches to benzene
The double bond breaks and partial +ve occur on the secondary carbon.
It attaches to benzene
What are the combustion products of benzene?- a)Carbon dioxide and water
- b)Carbon monoxide and oxygen
- c)Methane and nitrogen
- d)Hydrogen and sulfur dioxide
Correct answer is option 'A'. Can you explain this answer?
What are the combustion products of benzene?
a)
Carbon dioxide and water
b)
Carbon monoxide and oxygen
c)
Methane and nitrogen
d)
Hydrogen and sulfur dioxide
|
|
Deepak Iyer answered |
When benzene undergoes combustion, it reacts with oxygen to produce carbon dioxide (CO2) and water (H2O) as the combustion products. This is a common reaction for hydrocarbons in the presence of sufficient oxygen.
Aromatic hydrocarbons are known to exhibit:- a)High solubility in water
- b)Low flammability
- c)Low vapor pressure
- d)Carcinogenic properties
Correct answer is option 'D'. Can you explain this answer?
Aromatic hydrocarbons are known to exhibit:
a)
High solubility in water
b)
Low flammability
c)
Low vapor pressure
d)
Carcinogenic properties
|
|
Deepak Iyer answered |
Some aromatic hydrocarbons, such as benzene, are known to possess carcinogenic properties. Prolonged exposure to these substances can increase the risk of developing certain types of cancers.
Which of the following statements accurately describes the structure of benzene?- a)It consists of a five-membered carbon ring with alternating single and double bonds.
- b)It consists of a six-membered carbon ring with only single bonds.
- c)It consists of a six-membered carbon ring with alternating single and double bonds.
- d)It consists of a seven-membered carbon ring with alternating single and double bonds.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements accurately describes the structure of benzene?
a)
It consists of a five-membered carbon ring with alternating single and double bonds.
b)
It consists of a six-membered carbon ring with only single bonds.
c)
It consists of a six-membered carbon ring with alternating single and double bonds.
d)
It consists of a seven-membered carbon ring with alternating single and double bonds.
|
|
Deepak Iyer answered |
Benzene has a hexagonal structure formed by six carbon atoms. The carbon-carbon bonds in benzene are neither fully single bonds nor double bonds. Instead, they exhibit a phenomenon called resonance, where the electrons are delocalized, creating a continuous ring of alternating single and double bonds.
Aromatic hydrocarbons are commonly used in the production of:- a)Cooking oil
- b)Pharmaceuticals
- c)Fertilizers
- d)Plastics
Correct answer is option 'B'. Can you explain this answer?
Aromatic hydrocarbons are commonly used in the production of:
a)
Cooking oil
b)
Pharmaceuticals
c)
Fertilizers
d)
Plastics
|
|
Deepak Iyer answered |
Aromatic hydrocarbons find extensive use in the production of pharmaceuticals. Many drugs, such as aspirin, antibiotics, and painkillers, are synthesized using aromatic compounds as starting materials.
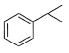
Direction (Q. Nos. 9 - 14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Which of the following com pound(s) fail in Friedel-Crafts alkylation reaction?- a)
- b)
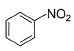
- c)
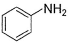
- d)
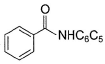
Correct answer is option 'B,C'. Can you explain this answer?
Direction (Q. Nos. 9 - 14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which of the following com pound(s) fail in Friedel-Crafts alkylation reaction?
a)
b)
c)
d)
|
|
Ishan Ghosh answered |
Nitrobenzene does not undergo Friedel craft reaction, because the nitro group of the nitrobenzene is a strong electron withdrawing group, and the oxygens in the nitro group are more electronegative and take away the electrons from the nitrogen forming electron deficient, which takes the electrons of the benzene ring, thereby deactivating it.
Friedel crafts reactions cannot occur on deactivated benzene (electron deficient), as the incoming electrophile repels from it.
Friedel crafts reactions cannot occur on deactivated benzene (electron deficient), as the incoming electrophile repels from it.
Direction (Q. Nos. 21 and 22) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.Q. Match the reactions from Column I with expected products from Column II.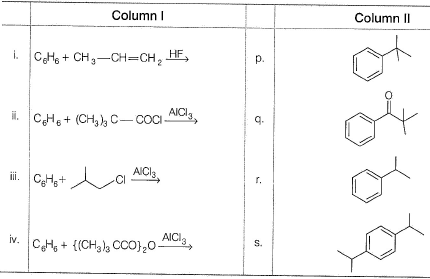

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 21 and 22) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q. Match the reactions from Column I with expected products from Column II.
a)
a
b)
b
c)
c
d)
d

|
Ashish Mishra answered |
D is correct
Which of the following is the molecular formula of benzene?- a)C6H6
- b)C6H10
- c)C5H5
- d)C7H8
Correct answer is option 'A'. Can you explain this answer?
Which of the following is the molecular formula of benzene?
a)
C6H6
b)
C6H10
c)
C5H5
d)
C7H8
|
|
Deepak Iyer answered |
Benzene consists of six carbon atoms (C6) and six hydrogen atoms (H6), giving it the molecular formula C6H6.
Which process is used to convert benzene to ethylbenzene?- a)Halogenation
- b)Nitration
- c)Alkylation
- d)Acylation
Correct answer is option 'C'. Can you explain this answer?
Which process is used to convert benzene to ethylbenzene?
a)
Halogenation
b)
Nitration
c)
Alkylation
d)
Acylation
|
|
Deepak Iyer answered |
Alkylation is the process used to convert benzene to ethylbenzene. It involves introducing an alkyl group (in this case, an ethyl group, C2H5) to the benzene ring through a substitution reaction.
Benzene is aromatic because it follows- a)Huckel’s rule
- b)Aromaticity conditions
- c)4n pi electron rule
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Benzene is aromatic because it follows
a)
Huckel’s rule
b)
Aromaticity conditions
c)
4n pi electron rule
d)
None of the above

|
Stepway Academy answered |
Explanation: According to the Huckel rule, compound should possess,
(i) Planarity
(ii) π electrons complete delocalisation in the ring
(iii) (4n + 2) π electrons presence in the ring where n is an integer (n = 0, 1, 2, . . .).
In the case of benzene, there are 6 pi electrons.
Hence, 4n+2 =6
This results in, n=1 (a positive value)
Because benzene follows Huckel’s rule, it is aromatic.
Which of the following compounds is an example of an aromatic hydrocarbon?- a)Ethane
- b)Propane
- c)Benzene
- d)Butane
Correct answer is option 'C'. Can you explain this answer?
Which of the following compounds is an example of an aromatic hydrocarbon?
a)
Ethane
b)
Propane
c)
Benzene
d)
Butane
|
|
Deepak Iyer answered |
Benzene is a six-membered aromatic hydrocarbon with a cyclic structure and delocalized pi electrons. It is the simplest and most well-known aromatic hydrocarbon.
Which of the following is an important industrial application of benzene?- a)Flavoring agent in food products
- b)Solvent for water-based paints
- c)Antacid in medicine
- d)Fuel for internal combustion engines
Correct answer is option 'A'. Can you explain this answer?
Which of the following is an important industrial application of benzene?
a)
Flavoring agent in food products
b)
Solvent for water-based paints
c)
Antacid in medicine
d)
Fuel for internal combustion engines
|
|
Deepak Iyer answered |
Benzene is not commonly used directly in food products due to its toxicity. However, it is present as a naturally occurring compound in some foods and is used indirectly as a starting material for the synthesis of flavoring agents that impart characteristic tastes and aromas to various food products.
Aromatic hydrocarbons are known for their:- a)High reactivity
- b)Low reactivity
- c)Alkaline nature
- d)Strong odor
Correct answer is option 'B'. Can you explain this answer?
Aromatic hydrocarbons are known for their:
a)
High reactivity
b)
Low reactivity
c)
Alkaline nature
d)
Strong odor
|
|
Deepak Iyer answered |
Aromatic hydrocarbons exhibit low reactivity due to the stability provided by the delocalized pi electrons in their cyclic structures. This stability makes them less prone to undergo chemical reactions compared to other hydrocarbons.
Consider the following reaction.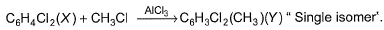 The correct statement(s) concerning X and Y is/are
The correct statement(s) concerning X and Y is/are- a)X is a non-polar!dichloride isomer
- b)X is more reactive than benzene in the same reaction
- c)Y gives three isomers of dimethylation product
- d)X and Y have different positions of chlorine ato
Correct answer is option 'A,C'. Can you explain this answer?
Consider the following reaction.
The correct statement(s) concerning X and Y is/are
a)
X is a non-polar!dichloride isomer
b)
X is more reactive than benzene in the same reaction
c)
Y gives three isomers of dimethylation product
d)
X and Y have different positions of chlorine ato

|
Ashish Mishra answered |
A and C is correct.
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Predict major product in the following reaction.
- a)
- b)
- c)
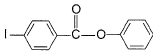
- d)
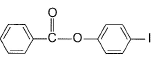
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Predict major product in the following reaction.
a)
b)
c)
d)
|
|
Love Jaiswy answered |
Group result in the left side would deactivate the left ring and oxygen would activate the right benzene ring through minus m effect so attack will be right benzene ring.
major major product will be para positioned as ortho position is hindered.
major major product will be para positioned as ortho position is hindered.
Which property differentiates aromatic hydrocarbons from aliphatic hydrocarbons?- a)Solubility in water
- b)Reactivity with oxygen
- c)Presence of double bonds
- d)Aromatic smell
Correct answer is option 'D'. Can you explain this answer?
Which property differentiates aromatic hydrocarbons from aliphatic hydrocarbons?
a)
Solubility in water
b)
Reactivity with oxygen
c)
Presence of double bonds
d)
Aromatic smell
|
|
Deepak Iyer answered |
Aromatic hydrocarbons are characterized by their distinct aromatic smell, whereas aliphatic hydrocarbons do not possess such odors. This odor is due to the presence of the aromatic ring in their structure.
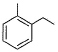
Direction (Q. Nos. 15 - 20) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IAn aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI. Q. The most likely structure of P is - a)
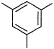
- b)
- c)
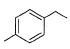
- d)
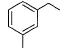
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 15 - 20) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
An aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI.
Q. The most likely structure of P is
a)
b)
c)
d)

|
Ashish Mishra answered |
D is correct.
Passage IAn aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI Q. Which of the following reaction represent best preparation of P?- a)
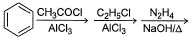
- b)
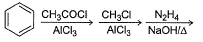
- c)
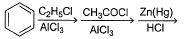
- d)
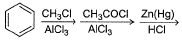
Correct answer is option 'B'. Can you explain this answer?
Passage I
An aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI
Q. Which of the following reaction represent best preparation of P?
a)
b)
c)
d)

|
Vijay Kolpe answered |
Just check out from options..
1st see which reaction can add 3 more carbon to benzen...so the option 1 get eliminated..and see the last step its of reduction and benzene contaion double bond..so it will perform wolf-kieshner rxn. ...option 3 and 4 eliminated..
no need to do rxn just calculate on bases of reagent..its less confusing and more helpful and time saving..
1st see which reaction can add 3 more carbon to benzen...so the option 1 get eliminated..and see the last step its of reduction and benzene contaion double bond..so it will perform wolf-kieshner rxn. ...option 3 and 4 eliminated..
no need to do rxn just calculate on bases of reagent..its less confusing and more helpful and time saving..
The main use of benzene is in the production of:- a)Pharmaceuticals
- b)Plastics
- c)Detergents
- d)Fertilizers
Correct answer is option 'B'. Can you explain this answer?
The main use of benzene is in the production of:
a)
Pharmaceuticals
b)
Plastics
c)
Detergents
d)
Fertilizers
|
|
Deepak Iyer answered |
Benzene is primarily used as a starting material in the production of various plastics. It serves as a precursor for the synthesis of polymers like polystyrene and polyethylene, which are widely used in the plastics industry.
What is /are the principal products of the following reaction ?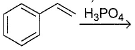
- a)
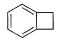
- b)
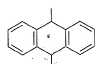
- c)
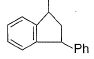
- d)
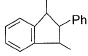
Correct answer is option 'B,C'. Can you explain this answer?
What is /are the principal products of the following reaction ?
a)
b)
c)
d)

|
Neet Bong Bong answered |
Here we have to proceed with the concept of electrophilic aromatic substitution
Chapter doubts & questions for Aromatic Hydrocarbons- Benzene - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Aromatic Hydrocarbons- Benzene - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup