All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Alkanols & Alkanones for JAMB Exam
Which reagent is commonly used in the Fehling's test to distinguish between alkanals and alkanones?- a)Sodium hydroxide
- b)Ammonium hydroxide
- c)Copper(II) sulfate
- d)Potassium iodide
Correct answer is option 'C'. Can you explain this answer?
Which reagent is commonly used in the Fehling's test to distinguish between alkanals and alkanones?
a)
Sodium hydroxide
b)
Ammonium hydroxide
c)
Copper(II) sulfate
d)
Potassium iodide
|
|
Deepak Iyer answered |
Fehling's test involves the use of Fehling's solution, which contains copper(II) sulfate. The alkanal or alkanone reacts with the copper(II) ions, leading to the reduction of copper(II) ions to copper(I) oxide, which appears as a colored precipitate.
Which chemical test is used to distinguish between an alkanal and an alkanone?- a)Tollen's test
- b)Baeyer's test
- c)Schiff's test
- d)Fehling's test
Correct answer is option 'D'. Can you explain this answer?
Which chemical test is used to distinguish between an alkanal and an alkanone?
a)
Tollen's test
b)
Baeyer's test
c)
Schiff's test
d)
Fehling's test
|
|
Deepak Iyer answered |
Fehling's test is commonly used to distinguish between alkanals (aldehydes) and alkanones (ketones). It involves the reaction of the carbonyl group with copper(II) ions, resulting in the formation of a colored precipitate.
In the reaction given below, 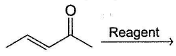 Q. which reagent(s) will bring about nucleophilic addition at carbonyl carbon?
Q. which reagent(s) will bring about nucleophilic addition at carbonyl carbon?- a)NH3
- b)HBr
- c)CH3MgBr
- d)CH3Li
Correct answer is option 'C,D'. Can you explain this answer?
In the reaction given below,
Q.
which reagent(s) will bring about nucleophilic addition at carbonyl carbon?
a)
NH3
b)
HBr
c)
CH3MgBr
d)
CH3Li

|
Adarsh Shukla answered |
Hard nucleophile are small ,charged, basic ,have low energy homo, like to attack c==o , example RO- , NH2- , methyl lithium
. Soft nucleophile are large ,neutral ,not basic ,high energy homo, like to attack saturated carbon. e.g. R3P, I-, RS-
Hence your option will be C ,D .
Which of the following is a general property of alkanals and alkanones?- a)High solubility in water
- b)High boiling points
- c)Low reactivity with oxidizing agents
- d)Non-flammable nature
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a general property of alkanals and alkanones?
a)
High solubility in water
b)
High boiling points
c)
Low reactivity with oxidizing agents
d)
Non-flammable nature
|
|
Deepak Iyer answered |
Alkanals and alkanones generally have higher boiling points compared to other organic compounds due to the presence of polar carbonyl groups. These groups increase intermolecular forces, resulting in higher boiling points.
Which of the following compounds would give a positive result in the 2,4-DNP test?- a)Butanone
- b)Methanol
- c)Ethanoic acid
- d)Ethanol
Correct answer is option 'A'. Can you explain this answer?
Which of the following compounds would give a positive result in the 2,4-DNP test?
a)
Butanone
b)
Methanol
c)
Ethanoic acid
d)
Ethanol
|
|
Deepak Iyer answered |
Butanone would give a positive result in the 2,4-DNP test, forming a colored precipitate. This test is specific for alkanones (ketones) and helps differentiate them from aldehydes.
In the Tollen's test, which compound would form a silver mirror?- a)Alkanal
- b)Alkanone
- c)Alkene
- d)Alkyne
Correct answer is option 'A'. Can you explain this answer?
In the Tollen's test, which compound would form a silver mirror?
a)
Alkanal
b)
Alkanone
c)
Alkene
d)
Alkyne
|
|
Deepak Iyer answered |
Alkanals (aldehydes) would form a silver mirror in the Tollen's test. The aldehyde group reduces silver ions to metallic silver, resulting in the formation of a silver mirror on the inner surface of the test tube.
Consider the following reaction, 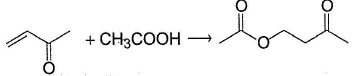 Q. The correct stalement(s) regarding mechanism of the above reaction and the reaction outcome is/are
Q. The correct stalement(s) regarding mechanism of the above reaction and the reaction outcome is/are- a)The indicated product is major one
- b)The reaction proceeds by nucleophilic attack of acid at protonated carbonyl at conjugate position
- c)Some aldol product is also formed
- d)Some .cyclic ester is also formed
Correct answer is option 'A,B'. Can you explain this answer?
Consider the following reaction,
Q.
The correct stalement(s) regarding mechanism of the above reaction and the reaction outcome is/are
a)
The indicated product is major one
b)
The reaction proceeds by nucleophilic attack of acid at protonated carbonyl at conjugate position
c)
Some aldol product is also formed
d)
Some .cyclic ester is also formed

|
Surbhi Sengupta answered |
CH3COOH undergoes nucleophilic addition at conjugate position giving indicated product as major one.
Comprehension TypeDirection (Q. Nos. 10-12) This section contains a paragraph, describing theory, experiments, data, etc.
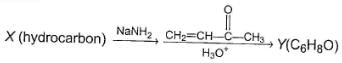
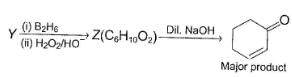
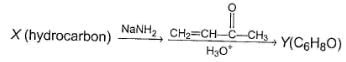
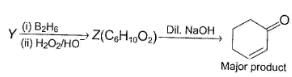
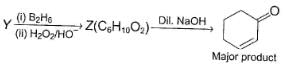
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageConsider the following multistep synthesis :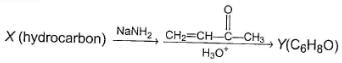
 Q. The hydrocarbon X is
Q. The hydrocarbon X is- a)C2H4
- b)C2H2
- c)
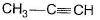
- d)
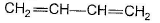
Correct answer is option 'B'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 10-12) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following multistep synthesis :


Q.
The hydrocarbon X is
a)
C2H4
b)
C2H2
c)
d)

|
Amar Pillai answered |
Y has 2 carbon extra than unsaturated ketone, hence starting compound must be acetylene and nucleophile undergoing conjugate addition is 
Only One Option Correct TypeDirection (Q. Nos. 1-5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Predict the major product in the given reaction, 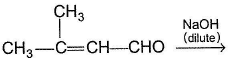
- a)
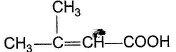
- b)
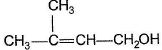
- c)
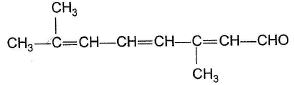
- d)Both (a) and (b)
Correct answer is option 'C'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Predict the major product in the given reaction, 
a)
b)
c)
d)
Both (a) and (b)

|
Ameya Basu answered |
The reaction of an alkanal or alkanone with 2,4-DNP reagent results in the formation of a colored precipitate. This test is known as:- a)Fehling's test
- b)Schiff's test
- c)Baeyer's test
- d)Brady's test
Correct answer is option 'B'. Can you explain this answer?
The reaction of an alkanal or alkanone with 2,4-DNP reagent results in the formation of a colored precipitate. This test is known as:
a)
Fehling's test
b)
Schiff's test
c)
Baeyer's test
d)
Brady's test
|
|
Deepak Iyer answered |
The reaction of an alkanal or alkanone with 2,4-DNP (2,4-dinitrophenylhydrazine) reagent forms a colored precipitate. This test is known as Schiff's test and is used to identify the presence of aldehydes and ketones.
Alkanals and alkanones are organic compounds that belong to which functional group?- a)Alkene
- b)Alkyne
- c)Aldehyde
- d)Carboxylic acid
Correct answer is option 'C'. Can you explain this answer?
Alkanals and alkanones are organic compounds that belong to which functional group?
a)
Alkene
b)
Alkyne
c)
Aldehyde
d)
Carboxylic acid
|
|
Deepak Iyer answered |
Alkanals and alkanones are organic compounds that contain the functional group known as the aldehyde group. Alkanals are aldehydes, while alkanones are ketones.
Which of the following is a major use of alkanals and alkanones?- a)Fuel for automobiles
- b)Food preservatives
- c)Plasticizers
- d)Antibiotics
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a major use of alkanals and alkanones?
a)
Fuel for automobiles
b)
Food preservatives
c)
Plasticizers
d)
Antibiotics
|
|
Deepak Iyer answered |
Alkanals and alkanones are commonly used as food preservatives to prevent spoilage. They have antimicrobial properties that inhibit the growth of bacteria and fungi in food products.
One or More than One Options Correct TypeDirection (Q. Nos. 6-9) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.In the reaction given below,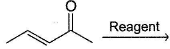 Q. which reagent(s) below would undergo addition at olefinic bond?
Q. which reagent(s) below would undergo addition at olefinic bond?- a)HCl
- b)NH3
- c)CH3MgBr
- d)NaH
Correct answer is option 'A,B'. Can you explain this answer?
One or More than One Options Correct Type
Direction (Q. Nos. 6-9) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
In the reaction given below,
Q.
which reagent(s) below would undergo addition at olefinic bond?
a)
HCl
b)
NH3
c)
CH3MgBr
d)
NaH

|
Amar Pillai answered |
Weak nucleophiles (Cl , NH3) undergo conjugate addition. Strong nucleophiles (R-, H-) undergo direct addition.
What is/are true about nucleophilic addition reaction at α, β-unsaturated carbonyl compound of type 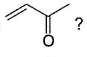
- a)LiAIH4 brings about addition at carbonyl carbon
- b)(CH3)2CuLi brings about addition at conjugate (olefinic) position
- c)CH3Li brings about conjugate (olefinic) addition
- d)CH3NH2 brings about conjugate (olefinic) addition
Correct answer is option 'A,B,D'. Can you explain this answer?
What is/are true about nucleophilic addition reaction at α, β-unsaturated carbonyl compound of type 
a)
LiAIH4 brings about addition at carbonyl carbon
b)
(CH3)2CuLi brings about addition at conjugate (olefinic) position
c)
CH3Li brings about conjugate (olefinic) addition
d)
CH3NH2 brings about conjugate (olefinic) addition

|
Ameya Basu answered |
Strong nucleophiles (like H- from LiAIH4) un dergo direct addition while weak nucleophiles (like R2CuLi, RNH2etc.) undergo conjugate addition at olefinic bond.
What is the systematic name for the alkanone with the molecular formula C₃H₆O?- a)Propanal
- b)Butanone
- c)Ethanal
- d)Pentanone
Correct answer is option 'B'. Can you explain this answer?
What is the systematic name for the alkanone with the molecular formula C₃H₆O?
a)
Propanal
b)
Butanone
c)
Ethanal
d)
Pentanone
|
|
Deepak Iyer answered |
Butanone is the systematic name for the alkanone with the molecular formula C₃H₆O. It is also known as methyl ethyl ketone (MEK).
One Integer Value Correct TypeDirection (Q. Nos. 13-15) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Q. In the reaction given below, how many different stereoisomers of major product are possible?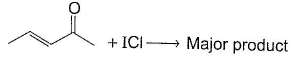
Correct answer is '4'. Can you explain this answer?
One Integer Value Correct Type
Direction (Q. Nos. 13-15) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
In the reaction given below, how many different stereoisomers of major product are possible?

|
Surbhi Sengupta answered |
What is a positive result for the Fehling's test?- a)Formation of a brick-red precipitate
- b)Formation of a silver mirror
- c)Evolution of a gas with a pungent smell
- d)Formation of a blue solution
Correct answer is option 'A'. Can you explain this answer?
What is a positive result for the Fehling's test?
a)
Formation of a brick-red precipitate
b)
Formation of a silver mirror
c)
Evolution of a gas with a pungent smell
d)
Formation of a blue solution
|
|
Deepak Iyer answered |
A positive result in the Fehling's test is the formation of a brick-red precipitate. This indicates the presence of an alkanal (aldehyde) or a reducing sugar, which can react with the copper(II) ions in Fehling's solution.
Chapter doubts & questions for Alkanols & Alkanones - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Alkanols & Alkanones - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily