All Exams >
Grade 6 >
Science for Grade 6 >
All Questions
All questions of Metals and Non-Metals for Grade 6 Exam
Gold : metal :: Silicon : _____a)non metalb)metalloidc)semi non metald)metalCorrect answer is option 'B'. Can you explain this answer?
|
|
Anita Menon answered |
Gold is metal and Silicon is a metalloid. Those elements that shows the property of both metal and non-metal are called metalloids.
When non-metals react with water then - a)Hydrogen gas is formed
- b)Carbon dioxide gas is formed
- c)Non-metals do not react with water
- d)None of these.
Correct answer is option 'C'. Can you explain this answer?
When non-metals react with water then
a)
Hydrogen gas is formed
b)
Carbon dioxide gas is formed
c)
Non-metals do not react with water
d)
None of these.
|
|
Amita Verma answered |
Non-metals don't react with water but the non-metal oxides do react with water and they produce acids. One example is chlorine gas (chlorine oxide) reacts with clouds (water) to form acids which come down as acid rain.
The metal alloyed with gold for making jewellery :- a)copper
- b)zinc
- c)silver
- d)aluminium
Correct answer is option 'A'. Can you explain this answer?
The metal alloyed with gold for making jewellery :
a)
copper
b)
zinc
c)
silver
d)
aluminium

|
Sanjana Bose answered |
Copper alloys are metal alloys that have copper as their principal component. They have high resistance against corrosion. The best known traditional types are bronze, where tin is a significant addition, and brass, using zinc instead.
Can you explain the answer of this question below:Metals are solid except :
- A:
mercury
- B:
gallium
- C:
sodium
- D:
iodine
The answer is A.
Metals are solid except :
mercury
gallium
sodium
iodine

|
Madhurima Nair answered |
All metals are found in solid state at normal room temperature except mercury, which is liquid at room temperature.
The property by which metals can be beaten into sheets is known as ___- a)Ductility
- b)Lusture
- c)Sonority
- d)Malleability
Correct answer is option 'D'. Can you explain this answer?
The property by which metals can be beaten into sheets is known as ___
a)
Ductility
b)
Lusture
c)
Sonority
d)
Malleability
|
|
Shubham Sharma answered |
Malleability is a physical property of metals that defines their ability to be hammered, pressed, or rolled into thin sheets without breaking. In other words, it is the property of a metal to deform under compression and take on a new shape.
Metalloids are also known as :- a)minerals
- b)semi metals
- c)impure metals
- d)alloys
Correct answer is option 'B'. Can you explain this answer?
Metalloids are also known as :
a)
minerals
b)
semi metals
c)
impure metals
d)
alloys

|
Ashwin Jain answered |
Metalloids, also known as semimetals are elements containing properties similar and midway between metals and nonmetals. They are found to divide the periodic table between the metals on the left and the nonmetals on the right. Metalloids often have the following properties: could be dull or shiny.
The metal which is stored in kerosene:- a)Phosphorus
- b)Magnesium
- c)Sodium
- d)Zinc.
Correct answer is option 'C'. Can you explain this answer?
The metal which is stored in kerosene:
a)
Phosphorus
b)
Magnesium
c)
Sodium
d)
Zinc.
|
|
Amit Sharma answered |
Because sodium catches fire in normal air .
Which of the following metals can be cut with a knife?- a)Aluminium
- b)Copper
- c)Sodium
- d)Calcium
Correct answer is option 'C'. Can you explain this answer?
Which of the following metals can be cut with a knife?
a)
Aluminium
b)
Copper
c)
Sodium
d)
Calcium
|
|
Kavya Saxena answered |
Metals like sodium and potassium are soft and can be cut with a knife.
A metal is characterised by :- a)non malleability
- b)high electrical conductivity
- c)low electrical conductivity
- d)non sonorosity
Correct answer is option 'B'. Can you explain this answer?
A metal is characterised by :
a)
non malleability
b)
high electrical conductivity
c)
low electrical conductivity
d)
non sonorosity
|
|
Pooja Shah answered |
Metal is characterised by high electrical conductivity. Metals are malleable, ductile, sonorous and good conductor of heat and electricity.
Aluminium foil is used for wrapping food. On which property is it used?- a)Density
- b)Malleability
- c)Ductility
- d)Strength
Correct answer is option 'B'. Can you explain this answer?
Aluminium foil is used for wrapping food. On which property is it used?
a)
Density
b)
Malleability
c)
Ductility
d)
Strength

|
Ishani Choudhury answered |
Aluminium foil is prepared by beating it into sheets, i.e., property of malleability. Hence, aluminium foil is used for the given application.
Chlorine is a very reactive metal.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
Chlorine is a very reactive metal.
a)
True
b)
False
|
|
Pooja Shah answered |
The chlorine is a very reactive now metal. All noble gases are considered as non - metals.
So, the given statement that chlorine is a very reactive metal, is false.
So, the given statement that chlorine is a very reactive metal, is false.
That property of a metal by virtue of which it can be drawn into wires is - a)malleability
- b)conductivity
- c)density
- d)ductility
Correct answer is option 'D'. Can you explain this answer?
That property of a metal by virtue of which it can be drawn into wires is
a)
malleability
b)
conductivity
c)
density
d)
ductility
|
|
Ananya Das answered |
The property of a metal by virtue of which metal can be drawn into thin wires is called ductility. Gold is the most ductile metal.
Rust formation is called :- a)deformation
- b)aberration
- c)corrosion
- d)illusion
Correct answer is 'C'. Can you explain this answer?
Rust formation is called :
a)
deformation
b)
aberration
c)
corrosion
d)
illusion
|
|
Pooja Shah answered |
Formation of rust on metal surface like iron is also called as corrosion. Corrosion occurs in presence of water and oxygen.
Bromine is only a liquid metal. - a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
Bromine is only a liquid metal.
a)
True
b)
False
|
|
Amit Sharma answered |
The correct option is B.
bromine is a fairly abundant element but has a rare property: it is the only nonmetal to exist in liquid form at room temperature, and one of only two elements (the other being mercury) that is liquid at room temperature and pressure.
bromine is a fairly abundant element but has a rare property: it is the only nonmetal to exist in liquid form at room temperature, and one of only two elements (the other being mercury) that is liquid at room temperature and pressure.
Can you explain the answer of this question below:Gas evolved when Calcium oxide reacts with water is/are :- A:oxygen
- B:carbon dioxide
- C:hydrogen
- D:both hydrogen & oxygen
The answer is c.
Gas evolved when Calcium oxide reacts with water is/are :
A:
oxygen
B:
carbon dioxide
C:
hydrogen
D:
both hydrogen & oxygen
|
|
Geetika Shah answered |
Solution :
The correct option is Option C.
The calcium metal begins to bubble vigorously as it reacts with the water, producing hydrogen gas, and a cloudy white precipitate of calcium hydroxide.
The metal which can form a barrier layer of oxide on its surface is :- a)Sodium
- b)Aluminium
- c)Carbon
- d)Barium
Correct answer is option 'B'. Can you explain this answer?
The metal which can form a barrier layer of oxide on its surface is :
a)
Sodium
b)
Aluminium
c)
Carbon
d)
Barium
|
|
Geetika Shah answered |
Aluminium form a barrier layer of oxide on its surface which prevents the further corrosion of aluminium on heating in presence of water.
Non-metal can be converted into wires.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
Non-metal can be converted into wires.
a)
True
b)
False
|
|
Geetika Shah answered |
Non- metals are non - (malleable and ductile). They are very brittle, and cannot be rolled into wires or poundes into sheets.
Non-metals are:- a)generally liquids
- b)generally gases
- c)generally solids and gases
- d)generally gases and liquids.
Correct answer is option 'C'. Can you explain this answer?
Non-metals are:
a)
generally liquids
b)
generally gases
c)
generally solids and gases
d)
generally gases and liquids.
|
|
Ritu Joshi answered |
Seventeen elements are generally classified as nonmetals; most are gases (hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypton, xenon and radon); one is a liquid (bromine), and a few are solids (carbon, phosphorus, sulfur, selenium, and iodine).
Metal oxides are :- a)basic oxides
- b)acidic oxides
- c)meteoroids
- d)neutral oxides
Correct answer is option 'A'. Can you explain this answer?
Metal oxides are :
a)
basic oxides
b)
acidic oxides
c)
meteoroids
d)
neutral oxides
|
|
Geetika Shah answered |
Metal oxides are basic in nature. When metal oxides react with water, they form bases. Water soluble bases are called s alkalis.
Metals produce basic oxide with oxygen.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
Metals produce basic oxide with oxygen.
a)
True
b)
False
|
|
Geetika Shah answered |
The correct option is A.
Oxides of metals are actually basic in nature not alkaline. This can be explained as oxygen reacts with metal to form corresponding metal oxides which are basic in nature. Also these metal oxides react with acid to form salt and water this shows that they are basic in nature.
Oxides of metals are actually basic in nature not alkaline. This can be explained as oxygen reacts with metal to form corresponding metal oxides which are basic in nature. Also these metal oxides react with acid to form salt and water this shows that they are basic in nature.
Non metal that conducts electricity is :- a)Iodine
- b)Coal
- c)Diamond
- d)Graphite
Correct answer is option 'D'. Can you explain this answer?
Non metal that conducts electricity is :
a)
Iodine
b)
Coal
c)
Diamond
d)
Graphite
|
|
Geetika Shah answered |
Non-metal are generally bad conductors of electricity but Graphite is an allotrope of carbon which is a good conductor of electricity.
The liquid metal at room temperature is:- a)Mercury
- b)Bromine
- c)Sodium
- d)Gold.
Correct answer is option 'A'. Can you explain this answer?
The liquid metal at room temperature is:
a)
Mercury
b)
Bromine
c)
Sodium
d)
Gold.
|
|
Shubham Sharma answered |
The correct option is A.
Liquid metal consists of alloys with very low melting points which form a eutectic that is liquid at room temperature. The standard metal used to be mercury, but gallium-based alloys, which are lower both in their vapor pressure at room temperature and toxicity, are being used as a replacement in various applications.
Liquid metal consists of alloys with very low melting points which form a eutectic that is liquid at room temperature. The standard metal used to be mercury, but gallium-based alloys, which are lower both in their vapor pressure at room temperature and toxicity, are being used as a replacement in various applications.
Sodium is such a hard metal that it cannot be cut with a knife.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
Sodium is such a hard metal that it cannot be cut with a knife.
a)
True
b)
False
|
|
Kavya Saxena answered |
Sodium is a soft metal that can be cut easily with a table knife. It is not a hard metal. Freshly cut sodium metal has a bright, shiny surface that quickly becomes a dull gray as it reacts with oxygen in the air around it.
So, the given statement is false.
So, the given statement is false.
Metal are hard but _____ can be cut with a knife- a)iron
- b)potassium
- c)mercury
- d)magnesium
Correct answer is option 'B'. Can you explain this answer?
Metal are hard but _____ can be cut with a knife
a)
iron
b)
potassium
c)
mercury
d)
magnesium

|
Pritam Sen answered |
Most of the metals are hard but potassium is very soft metal which can be cut with knife. It is stored in kerosene oil to prevent accidental fire.
If metallic Zinc is added to copper sulphate solution :- a)Zinc displaces copper from solution
- b)no reaction takes place
- c)Sulphur is displaced
- d)Copper displaces Zinc from solution
Correct answer is option 'A'. Can you explain this answer?
If metallic Zinc is added to copper sulphate solution :
a)
Zinc displaces copper from solution
b)
no reaction takes place
c)
Sulphur is displaced
d)
Copper displaces Zinc from solution

|
Anu Choudhury answered |
Z
n
+CuSO4→ZnSO4 +Cu.Zn +CuSO4→ZnSO4 +Cu.If Zinc is added to copper sulphate solution, Copper is displaced by Zinc because zinc is more reactive than copper.The non-metal which is liquid at room temperature is:- a)Carbon
- b)Iodine
- c)Bromine
- d)Chlorine.
Correct answer is option 'C'. Can you explain this answer?
The non-metal which is liquid at room temperature is:
a)
Carbon
b)
Iodine
c)
Bromine
d)
Chlorine.

|
Priya Nair answered |
For example, carbon, sulphur and phosphorus are solid at room temperature. Nitrogen and oxygen are gaseous non-metals. Bromine is a non-metal which is liquid at room temperature.
Non metals occur as:- a)gas
- b)solid
- c)liquid
- d)all of these
Correct answer is option 'D'. Can you explain this answer?
Non metals occur as:
a)
gas
b)
solid
c)
liquid
d)
all of these

|
Divya Patel answered |
Non-metals are found in nature in solid, liquid and gaseous state. On the other hand metals are found in solid state except mercury that is liquid at room temperature.
Sodium and potassium are kept in water.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
Sodium and potassium are kept in water.
a)
True
b)
False
|
|
Ananya Das answered |
Sodium and potassium metals reacts very vigorously with water ; this reaction gives out so much heat that the hydrogen evolved catches fire. Therefore, these metals are stored under kerosene .
So, the given statement is false.
So, the given statement is false.
How many electrons are present in non- metals in their outermost shell?- a)1, 2 or 3
- b)8, 9 or 10
- c)10, 20 or 30
- d)4, 5, 6 or 7
Correct answer is option 'D'. Can you explain this answer?
How many electrons are present in non- metals in their outermost shell?
a)
1, 2 or 3
b)
8, 9 or 10
c)
10, 20 or 30
d)
4, 5, 6 or 7

|
Sahil Singh answered |
Non-metals generally contain 4,5,6,7 or 8 electrons in their outermost orbit or shell.
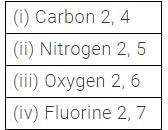
The number of valence electrons in carbon, nitrogen, oxygen and fluorine are 4, 5, 6, 7 respectively.
Zinc reacts with hydrochloric acid to form :- a)Hydrogen gas
- b)Zinc Chloride
- c)both Zinc Chloride & Hydrogen gas
- d)Oxygen gas
Correct answer is option 'C'. Can you explain this answer?
Zinc reacts with hydrochloric acid to form :
a)
Hydrogen gas
b)
Zinc Chloride
c)
both Zinc Chloride & Hydrogen gas
d)
Oxygen gas

|
Anu Choudhury answered |
Zinc reacts with hydrochloric acid to form zinc chloride with evolution of hydrogen gas. Zn+2HCl→ZnCl2+ H2
Which non-metal is used in the treatment of rubber during the process of vulcani-sation?- a)Sulphur
- b)Phosphorus
- c)arbon
- d)Chlorine
Correct answer is option 'A'. Can you explain this answer?
Which non-metal is used in the treatment of rubber during the process of vulcani-sation?
a)
Sulphur
b)
Phosphorus
c)
arbon
d)
Chlorine

|
Anoushka Das answered |
Sulphur is used in the process of vulcanization. Rubber when treated with sulphur becomes harder than ordinary rubber, so it is used to make tyres.
Metal oxides are of nature- a)Acidic
- b)Basic
- c)Neutral
- d)All of these.
Correct answer is option 'B'. Can you explain this answer?
Metal oxides are of nature
a)
Acidic
b)
Basic
c)
Neutral
d)
All of these.
|
|
🌸Shailvi Srivastava🌸 answered |
Metal oxides are always basic in nature
Metals that react with both acids and bases are called :- a)amphibian
- b)amphiteric
- c)amphitamine
- d)amphoteric
Correct answer is option 'D'. Can you explain this answer?
Metals that react with both acids and bases are called :
a)
amphibian
b)
amphiteric
c)
amphitamine
d)
amphoteric

|
Sanjana Bose answered |
Metal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides. Amphoteric oxides include lead oxide and zinc oxide, among many others.
Which metal reacts vigorously with HCl to produce salt and hydrogen?- a)Na
- b)Zn
- c)Sn
- d)Pb
Correct answer is option 'A'. Can you explain this answer?
Which metal reacts vigorously with HCl to produce salt and hydrogen?
a)
Na
b)
Zn
c)
Sn
d)
Pb

|
Akshara Iyer answered |
Among the given metals, sodium is a highly reactive metal, so it reacts vigorously to form salt and hydrogen.
2Na+2HCl→2NaCl+H2↑
Metals are solid except :- a)mercury
- b)gallium
- c)sodium
- d)iodine
Correct answer is option 'A'. Can you explain this answer?
Metals are solid except :
a)
mercury
b)
gallium
c)
sodium
d)
iodine

|
Sounak Sengupta answered |
Mercury is the only metal that is a liquid at normal temperatures and pressure. Most metal atoms readily share valence electrons with other atoms. The electrons in a mercury atom are bound more tightly than usual to the nucleus.
It takes very little heat to overcome the weak binding between mercury atoms. Because of the behavior of the valence electrons, mercury has a low melting point, is a poor electrical and thermal conductor and doesn't form diatomic mercury molecules in the gas phase.
When Silver is added to Copper Nitrate solution result is :- a)no reaction
- b)formation of Silver Nitrate
- c)formation of alloy
- d)formation Silver Nitrite
Correct answer is option 'A'. Can you explain this answer?
When Silver is added to Copper Nitrate solution result is :
a)
no reaction
b)
formation of Silver Nitrate
c)
formation of alloy
d)
formation Silver Nitrite

|
Pritam Sen answered |
When silver is added to copper nitrate solution, no reaction takes place because silver is less reactive than copper.
Identify the metal that replaces magnesium from its salt.- a)Ca
- b)Al
- c)Zn
- d)Fe
Correct answer is option 'A'. Can you explain this answer?
Identify the metal that replaces magnesium from its salt.
a)
Ca
b)
Al
c)
Zn
d)
Fe

|
Priya Chakraborty answered |
Calcium is more reactive than magnesium. Hence, Mg can be replaced from its salt by calcium.
Why is tungsten used as a filament in electric bulbs?- a)It is sonorous.
- b)It is metallic.
- c)It has a high melting point.
- d)It has high density
Correct answer is option 'C'. Can you explain this answer?
Why is tungsten used as a filament in electric bulbs?
a)
It is sonorous.
b)
It is metallic.
c)
It has a high melting point.
d)
It has high density

|
Divya Patel answered |
Metal tungsten is used as a filament in electric bulbs because it has a high melting point.
Brass is an alloy of :- a)zinc – copper- tin
- b)copper – tin
- c)zinc – copper
- d)zinc – tin
Correct answer is option 'C'. Can you explain this answer?
Brass is an alloy of :
a)
zinc – copper- tin
b)
copper – tin
c)
zinc – copper
d)
zinc – tin
|
|
Sarita Verma answered |
Brass is a metallic alloy that is made of copper and zinc. The proportions of zinc and copper can vary to create different types of brass alloys with varying mechanical and electrical properties. It is a substitutional alloy: atoms of the two constituents may replace each other within the same crystal structure.
Oxides of non-metals are :- a)absolute amphoteric
- b)metalloid
- c)acidic
- d)basic
Correct answer is option 'C'. Can you explain this answer?
Oxides of non-metals are :
a)
absolute amphoteric
b)
metalloid
c)
acidic
d)
basic
|
|
Anirudh Sengupta answered |
Non-metals are a group of elements on the periodic table that have properties opposite to those of metals. One characteristic of non-metals is their tendency to form oxides when they react with oxygen. Oxides of non-metals are generally acidic in nature. This means that when they dissolve in water, they produce hydrogen ions (H+) which can react with bases to form water and a salt.
Explanation:
1. What are oxides of non-metals?
- Oxides of non-metals are compounds that are formed when non-metal elements combine with oxygen.
2. Acidic nature of non-metal oxides:
- Non-metal oxides are generally acidic in nature. This is because non-metals have a high electronegativity, meaning they attract electrons strongly.
- When non-metals react with oxygen, they gain electrons and form negatively charged ions (anions) such as O2-, N3-, and S2-.
- These anions can combine with hydrogen ions (H+) in water to form acids.
- For example, sulfur dioxide (SO2) is a non-metal oxide that dissolves in water to form sulfurous acid (H2SO3). The hydrogen ions from the acid can react with bases to form water and a salt.
3. Examples of acidic non-metal oxides:
- Carbon dioxide (CO2): When dissolved in water, it forms carbonic acid (H2CO3).
- Nitrogen dioxide (NO2): Dissolves in water to form nitric acid (HNO3).
- Sulfur dioxide (SO2): Reacts with water to form sulfurous acid (H2SO3).
4. Importance of acidic non-metal oxides:
- Acidic non-metal oxides play important roles in various natural processes and human activities.
- For example, carbon dioxide is a greenhouse gas that contributes to climate change.
- Sulfur dioxide is released during the combustion of fossil fuels and is a major contributor to air pollution and acid rain.
In conclusion, oxides of non-metals are generally acidic in nature. They can dissolve in water to form acids, which can react with bases to produce water and a salt. This characteristic is due to the high electronegativity of non-metals, which allows them to attract electrons and form negatively charged ions.
Explanation:
1. What are oxides of non-metals?
- Oxides of non-metals are compounds that are formed when non-metal elements combine with oxygen.
2. Acidic nature of non-metal oxides:
- Non-metal oxides are generally acidic in nature. This is because non-metals have a high electronegativity, meaning they attract electrons strongly.
- When non-metals react with oxygen, they gain electrons and form negatively charged ions (anions) such as O2-, N3-, and S2-.
- These anions can combine with hydrogen ions (H+) in water to form acids.
- For example, sulfur dioxide (SO2) is a non-metal oxide that dissolves in water to form sulfurous acid (H2SO3). The hydrogen ions from the acid can react with bases to form water and a salt.
3. Examples of acidic non-metal oxides:
- Carbon dioxide (CO2): When dissolved in water, it forms carbonic acid (H2CO3).
- Nitrogen dioxide (NO2): Dissolves in water to form nitric acid (HNO3).
- Sulfur dioxide (SO2): Reacts with water to form sulfurous acid (H2SO3).
4. Importance of acidic non-metal oxides:
- Acidic non-metal oxides play important roles in various natural processes and human activities.
- For example, carbon dioxide is a greenhouse gas that contributes to climate change.
- Sulfur dioxide is released during the combustion of fossil fuels and is a major contributor to air pollution and acid rain.
In conclusion, oxides of non-metals are generally acidic in nature. They can dissolve in water to form acids, which can react with bases to produce water and a salt. This characteristic is due to the high electronegativity of non-metals, which allows them to attract electrons and form negatively charged ions.
Which material is used for making crucibles?- a)Sulphur
- b)Silicon
- c)Graphite
- d)Phosphorus
Correct answer is option 'C'. Can you explain this answer?
Which material is used for making crucibles?
a)
Sulphur
b)
Silicon
c)
Graphite
d)
Phosphorus

|
Shivani Iyer answered |
Crucibles are used in the laboratory to perform high temperature reactions. They should resist high heating (they should not break). For this purpose, a mixture of graphite (an allotrope of carbon) and clay is used to make crucibles.
Identify the metal that does NOT react with HCl
- a)Copper and Mercury
- b)Magnesium
- c)Zinc
- d)Iron
Correct answer is option 'A'. Can you explain this answer?
Identify the metal that does NOT react with HCl
a)
Copper and Mercury
b)
Magnesium
c)
Zinc
d)
Iron

|
Priya Chakraborty answered |
Copper and mercury metal do not react with dilute hydrochloric acid as they come after hydrogen in the activity series, i.e., they can't replace hydrogen from hydrochloric acid.
Calcium disappears into water forming :- a)Calcium carbonate
- b)transparent water
- c)oxygen gas
- d)milky water
Correct answer is option 'D'. Can you explain this answer?
Calcium disappears into water forming :
a)
Calcium carbonate
b)
transparent water
c)
oxygen gas
d)
milky water

|
Samridhi Banerjee answered |
When calcium hydroxide (lime water) is passed through carbon dioxide gas water turns milky due to formation of calcium carbonate, which is soluble in water.
The metal which can be cut with a knife- a)Sodium and potassium
- b)Barium and calcium
- c)Sodium and mercury
- d)Potassium and calcium.
Correct answer is option 'A'. Can you explain this answer?
The metal which can be cut with a knife
a)
Sodium and potassium
b)
Barium and calcium
c)
Sodium and mercury
d)
Potassium and calcium.
|
|
Ananya Das answered |
Sodium and potassium are soft metals. So an ordinary knife and a little pressure is enough to cut them.
Metals are usually ____ and good conductors, forming basic oxides.- a)dull
- b)lustrous
- c)brittle
- d)malleable
Correct answer is option 'B'. Can you explain this answer?
Metals are usually ____ and good conductors, forming basic oxides.
a)
dull
b)
lustrous
c)
brittle
d)
malleable
|
|
Ishita Mehta answered |
Understanding Metals
Metals are an essential category of elements that possess unique properties. Among these properties, luster is particularly significant, making option 'B' the correct answer.
Key Properties of Metals:
- Luster: Metals typically exhibit a shiny appearance due to their ability to reflect light. This characteristic is often described as "lustrous." It is a defining feature that distinguishes metals from non-metals, which usually appear dull.
- Malleability: Many metals can be easily shaped or hammered into thin sheets. This property allows metals to be used in various applications, from construction materials to jewelry making.
- Ductility: Metals can also be drawn into wires without breaking. This property is crucial for electrical conduction and wiring applications.
- Good Conductors: Metals are excellent conductors of heat and electricity. This makes them ideal for use in electrical appliances, wiring, and heat exchangers.
- Formation of Basic Oxides: When metals react with oxygen, they often form basic oxides. For example, magnesium oxide and aluminum oxide are formed from their respective metals.
Conclusion:
In summary, metals are generally lustrous, malleable, and good conductors, which explains why option 'B' is the correct choice. Their shiny appearance not only defines their identity but also plays a critical role in their various applications across different industries. Understanding these properties helps in recognizing the importance of metals in our daily lives.
Metals are an essential category of elements that possess unique properties. Among these properties, luster is particularly significant, making option 'B' the correct answer.
Key Properties of Metals:
- Luster: Metals typically exhibit a shiny appearance due to their ability to reflect light. This characteristic is often described as "lustrous." It is a defining feature that distinguishes metals from non-metals, which usually appear dull.
- Malleability: Many metals can be easily shaped or hammered into thin sheets. This property allows metals to be used in various applications, from construction materials to jewelry making.
- Ductility: Metals can also be drawn into wires without breaking. This property is crucial for electrical conduction and wiring applications.
- Good Conductors: Metals are excellent conductors of heat and electricity. This makes them ideal for use in electrical appliances, wiring, and heat exchangers.
- Formation of Basic Oxides: When metals react with oxygen, they often form basic oxides. For example, magnesium oxide and aluminum oxide are formed from their respective metals.
Conclusion:
In summary, metals are generally lustrous, malleable, and good conductors, which explains why option 'B' is the correct choice. Their shiny appearance not only defines their identity but also plays a critical role in their various applications across different industries. Understanding these properties helps in recognizing the importance of metals in our daily lives.
Chapter doubts & questions for Metals and Non-Metals - Science for Grade 6 2025 is part of Grade 6 exam preparation. The chapters have been prepared according to the Grade 6 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 6 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Metals and Non-Metals - Science for Grade 6 in English & Hindi are available as part of Grade 6 exam.
Download more important topics, notes, lectures and mock test series for Grade 6 Exam by signing up for free.
Science for Grade 6
101 videos|166 docs|51 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup











