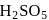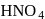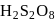All Exams >
Grade 9 >
Chemistry for Grade 9 >
All Questions
All questions of Sulphur for Grade 9 Exam
Which of the following is the chemical symbol for sulphur?- a)S
- b)Su
- c)Sr
- d)Se
Correct answer is option 'A'. Can you explain this answer?
Which of the following is the chemical symbol for sulphur?
a)
S
b)
Su
c)
Sr
d)
Se

|
Sun Ray Institute answered |
The chemical symbol for sulphur is "S" in the periodic table.
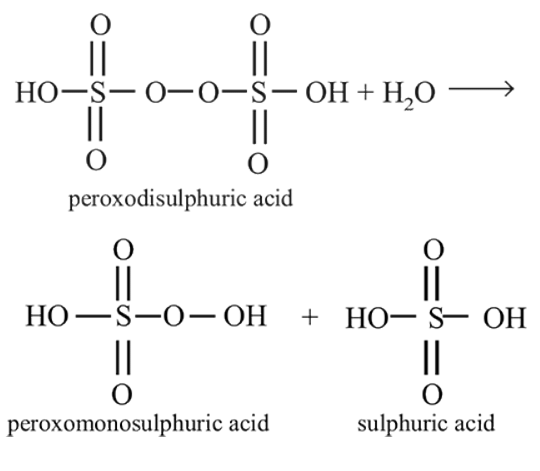
Hydrolysis of one mole of peroxodisulphuric acid produces- a)two moles of sulphuric acid.
- b)two moles of peroxomonosulphuric acid.
- c)one mole of sulphuric acid and one mole of peroxomonosulphuric acid.
- d)one mole of sulphuric acid, one mole of peroxomonosulphuric acid and one mole of hydrogen peroxide.
Correct answer is option 'C'. Can you explain this answer?
Hydrolysis of one mole of peroxodisulphuric acid produces
a)
two moles of sulphuric acid.
b)
two moles of peroxomonosulphuric acid.
c)
one mole of sulphuric acid and one mole of peroxomonosulphuric acid.
d)
one mole of sulphuric acid, one mole of peroxomonosulphuric acid and one mole of hydrogen peroxide.

|
Imk Pathsala answered |
Sulphur is an important component in which type of rocks?- a)Igneous rocks
- b)Sedimentary rocks
- c)Metamorphic rocks
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Sulphur is an important component in which type of rocks?
a)
Igneous rocks
b)
Sedimentary rocks
c)
Metamorphic rocks
d)
None of the above

|
Sun Ray Institute answered |
Sulphur is commonly found in sedimentary rocks, including gypsum and pyrite.
The number of  bonds in
bonds in 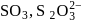
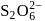 and
and 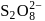 respectively are
respectively are- a)
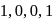
- b)
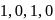
- c)
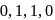
- d)
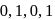
Correct answer is option 'C'. Can you explain this answer?
The number of  bonds in
bonds in 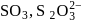
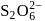 and
and 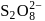 respectively are
respectively are
 bonds in
bonds in 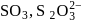
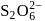 and
and 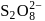 respectively are
respectively area)
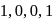
b)
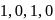
c)
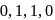
d)
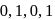

|
Tarun Kaushik answered |
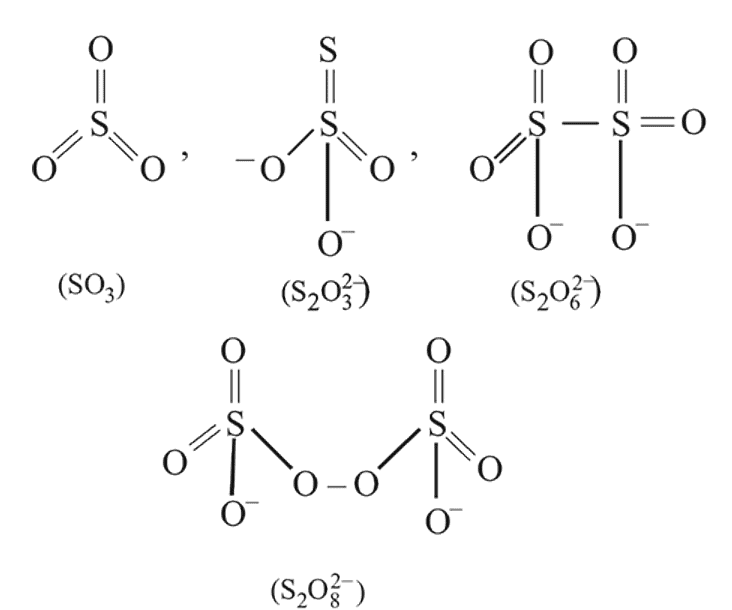
Hence (c) is the correct option.
Which of the following substances has a characteristic smell similar to rotten eggs?- a)Sulphuric acid
- b)Sulphur dioxide
- c)Sulphur hexafluoride
- d)Sodium sulphate
Correct answer is option 'B'. Can you explain this answer?
Which of the following substances has a characteristic smell similar to rotten eggs?
a)
Sulphuric acid
b)
Sulphur dioxide
c)
Sulphur hexafluoride
d)
Sodium sulphate

|
Sun Ray Institute answered |
Sulphur dioxide has a pungent odor similar to that of rotten eggs.
Which of the following is a common use of sulphur?- a)Battery production
- b)Fuel for cars
- c)Building materials
- d)Fertilizer manufacturing
Correct answer is option 'D'. Can you explain this answer?
Which of the following is a common use of sulphur?
a)
Battery production
b)
Fuel for cars
c)
Building materials
d)
Fertilizer manufacturing

|
Sun Ray Institute answered |
Sulphur is widely used in the production of fertilizers, particularly as a component of sulphuric acid.
Sulfur dioxide (SO2) is commonly used in which industry?- a)Textile manufacturing
- b)Food preservation
- c)Semiconductor production
- d)Glassmaking
Correct answer is option 'D'. Can you explain this answer?
Sulfur dioxide (SO2) is commonly used in which industry?
a)
Textile manufacturing
b)
Food preservation
c)
Semiconductor production
d)
Glassmaking

|
Sun Ray Institute answered |
Sulfur dioxide (SO2) is used in the glassmaking industry. It acts as a reducing agent and helps remove impurities from the molten glass, resulting in clearer and more transparent glass.
What is the use of sulfur in vulcanization?- a)Preserving food
- b)Enhancing plant growth
- c)Making explosives
- d)Strengthening rubber
Correct answer is option 'D'. Can you explain this answer?
What is the use of sulfur in vulcanization?
a)
Preserving food
b)
Enhancing plant growth
c)
Making explosives
d)
Strengthening rubber

|
Sun Ray Institute answered |
Sulfur is utilized in the vulcanization process, which strengthens rubber by forming cross-links between polymer chains. Vulcanized rubber exhibits improved elasticity, durability, and resistance to heat and abrasion.
Which of the following is not an allotrope of sulfur?- a)Rhombic sulfur
- b)Monoclinic sulfur
- c)Amorphous sulfur
- d)Graphite sulfur
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not an allotrope of sulfur?
a)
Rhombic sulfur
b)
Monoclinic sulfur
c)
Amorphous sulfur
d)
Graphite sulfur

|
Sun Ray Institute answered |
Sulfur has several allotropes, including rhombic sulfur, monoclinic sulfur, and amorphous sulfur. However, graphite sulfur is not an allotrope of sulfur. Graphite is a form of carbon and does not contain sulfur.
Which of the following is incorrect?- a)M.p of monoclinic sulphur
 m.p. of rhombic sulphur.
m.p. of rhombic sulphur. - b)Specific gravity of rhombic sulphur
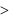 specific gravity of monoclinic sulphur.
specific gravity of monoclinic sulphur. - c)Monoclinic sulphur is stable below
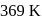 .
. - d)Both rhombic sulphur and monoclinic sulphur have
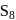 molecules.
molecules.
Correct answer is option 'C'. Can you explain this answer?
a)
M.p of monoclinic sulphur 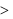 m.p. of rhombic sulphur.
m.p. of rhombic sulphur.
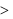 m.p. of rhombic sulphur.
m.p. of rhombic sulphur.b)
Specific gravity of rhombic sulphur 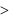 specific gravity of monoclinic sulphur.
specific gravity of monoclinic sulphur.
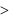 specific gravity of monoclinic sulphur.
specific gravity of monoclinic sulphur.c)
Monoclinic sulphur is stable below 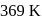 .
.
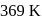 .
.d)
Both rhombic sulphur and monoclinic sulphur have 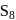 molecules.
molecules.
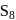 molecules.
molecules.

|
Manish Aggarwal answered |
Monoclinic sulphur is stable above 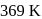 .
.
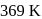 .
.Which of the following is the key step in the manufacture of sulphuric acid?- a)Burning of sulphur or sulphide ores in air to generate
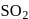 .
. - b)Conversion of
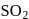 to
to 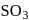 by the reaction with oxygen in presence of catalyst.
by the reaction with oxygen in presence of catalyst. - c)Absorption of
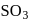 in
in 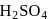 to give oleum.
to give oleum. - d)Both (b) and (c).
Correct answer is option 'B'. Can you explain this answer?
a)
Burning of sulphur or sulphide ores in air to generate 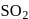 .
.
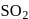 .
.b)
Conversion of 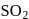 to
to 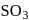 by the reaction with oxygen in presence of catalyst.
by the reaction with oxygen in presence of catalyst.
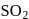 to
to 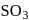 by the reaction with oxygen in presence of catalyst.
by the reaction with oxygen in presence of catalyst.c)
Absorption of 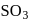 in
in 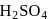 to give oleum.
to give oleum.
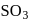 in
in 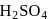 to give oleum.
to give oleum.d)
Both (b) and (c).

|
Manish Aggarwal answered |
The key step in the manufacture of 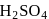 is catalytic oxidation of
is catalytic oxidation of 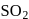 with
with 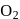 to give
to give 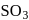 in presence of
in presence of 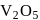 .
.
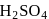 is catalytic oxidation of
is catalytic oxidation of 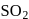 with
with 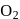 to give
to give 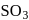 in presence of
in presence of 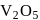 .
.Which allotrope of sulfur is also known as "plastic sulfur"?- a)Rhombic sulfur
- b)Monoclinic sulfur
- c)Amorphous sulfur
- d)Liquid sulfur
Correct answer is option 'C'. Can you explain this answer?
Which allotrope of sulfur is also known as "plastic sulfur"?
a)
Rhombic sulfur
b)
Monoclinic sulfur
c)
Amorphous sulfur
d)
Liquid sulfur

|
Sun Ray Institute answered |
Amorphous sulfur is commonly referred to as "plastic sulfur." It is a non-crystalline form of sulfur that can be easily molded or shaped when heated.
Sulphur is commonly found in which state at room temperature?- a)Solid
- b)Liquid
- c)Gas
- d)Plasma
Correct answer is option 'A'. Can you explain this answer?
Sulphur is commonly found in which state at room temperature?
a)
Solid
b)
Liquid
c)
Gas
d)
Plasma

|
Sun Ray Institute answered |
Sulphur exists as a yellow solid at room temperature and atmospheric pressure.
Which of the following has pπ−dπ bonding ?- a)
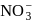
- b)
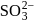
- c)
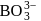
- d)
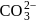
Correct answer is option 'B'. Can you explain this answer?
Which of the following has pπ−dπ bonding ?
a)
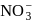
b)
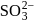
c)
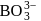
d)
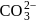

|
Learners Habitat answered |
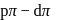 bonding is present in
bonding is present in 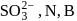 ,
,C have no vacant
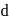 atomic arbitals.
atomic arbitals.
What is the primary use of sulfur in the production of sulfuric acid?- a)Fertilizer production
- b)Medicinal applications
- c)Battery manufacturing
- d)Industrial chemical synthesis
Correct answer is option 'D'. Can you explain this answer?
What is the primary use of sulfur in the production of sulfuric acid?
a)
Fertilizer production
b)
Medicinal applications
c)
Battery manufacturing
d)
Industrial chemical synthesis

|
Sun Ray Institute answered |
Sulfur is extensively used in the production of sulfuric acid, which is a vital industrial chemical. Sulfuric acid is used in various industrial processes such as the production of fertilizers, dyes, detergents, and pharmaceuticals.
Which of the following is a medicinal use of sulfur?- a)Treatment of acne
- b)Flavor enhancer
- c)Catalyst in chemical reactions
- d)Corrosion prevention
Correct answer is option 'A'. Can you explain this answer?
Which of the following is a medicinal use of sulfur?
a)
Treatment of acne
b)
Flavor enhancer
c)
Catalyst in chemical reactions
d)
Corrosion prevention

|
Sun Ray Institute answered |
Sulfur is used in the treatment of acne due to its antibacterial and keratolytic properties. It helps to control excess oil production, unclog pores, and reduce inflammation, thus aiding in the treatment of acne.
Which allotrope of sulfur is used in matches?- a)Rhombic sulfur
- b)Monoclinic sulfur
- c)Amorphous sulfur
- d)Liquid sulfur
Correct answer is option 'C'. Can you explain this answer?
Which allotrope of sulfur is used in matches?
a)
Rhombic sulfur
b)
Monoclinic sulfur
c)
Amorphous sulfur
d)
Liquid sulfur

|
Sun Ray Institute answered |
Amorphous sulfur, also known as plastic sulfur, is used in matchstick heads. When ignited, amorphous sulfur reacts with other components to create a flame.
Sulphur is an important element in which amino acid?- a)Methionine
- b)Glycine
- c)Leucine
- d)Serine
Correct answer is option 'A'. Can you explain this answer?
Sulphur is an important element in which amino acid?
a)
Methionine
b)
Glycine
c)
Leucine
d)
Serine

|
Sun Ray Institute answered |
Sulphur is present in the amino acid methionine, an essential amino acid required for protein synthesis.
Which allotrope of sulfur is thermodynamically stable at room temperature?- a)Rhombic sulfur
- b)Monoclinic sulfur
- c)Amorphous sulfur
- d)Liquid sulfur
Correct answer is option 'B'. Can you explain this answer?
Which allotrope of sulfur is thermodynamically stable at room temperature?
a)
Rhombic sulfur
b)
Monoclinic sulfur
c)
Amorphous sulfur
d)
Liquid sulfur

|
Sun Ray Institute answered |
Monoclinic sulfur is the thermodynamically stable allotrope of sulfur at room temperature. It is a dense, yellow crystalline form of sulfur and transitions to rhombic sulfur at temperatures above 96°C.
Which process is used to extract sulphur from underground deposits?- a)Smelting
- b)Electrolysis
- c)Fracking
- d)Sublimation
Correct answer is option 'A'. Can you explain this answer?
Which process is used to extract sulphur from underground deposits?
a)
Smelting
b)
Electrolysis
c)
Fracking
d)
Sublimation

|
Sun Ray Institute answered |
Sulphur is extracted from underground deposits through a process called smelting, which involves heating the sulphur-containing rocks to release the element.
Which of the following form of the sulphur shows paramagnetic behaviour?- a)
- b)
- c)
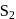
- d)All of these
Correct answer is option 'C'. Can you explain this answer?
a)
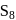
b)
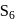
c)
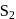
d)
All of these

|
Learners Habitat answered |
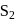 is paramagnetic. It contains two unpaired electrons in the antibonding
is paramagnetic. It contains two unpaired electrons in the antibonding  orbital
orbitalWhich of the following compounds is commonly used as a fungicide in agriculture?- a)Sulphur dioxide
- b)Sulphur hexafluoride
- c)Sulphuric acid
- d)Elemental sulphur
Correct answer is option 'D'. Can you explain this answer?
Which of the following compounds is commonly used as a fungicide in agriculture?
a)
Sulphur dioxide
b)
Sulphur hexafluoride
c)
Sulphuric acid
d)
Elemental sulphur

|
Sun Ray Institute answered |
Elemental sulphur is commonly used as a fungicide in agriculture to control various fungal diseases in crops and plants.
Which of the following compounds contains sulphur?- a)Water (H2O)
- b)Carbon dioxide (CO2)
- c)Sulphuric acid (H2SO4)
- d)Methane (CH4)
Correct answer is option 'C'. Can you explain this answer?
Which of the following compounds contains sulphur?
a)
Water (H2O)
b)
Carbon dioxide (CO2)
c)
Sulphuric acid (H2SO4)
d)
Methane (CH4)

|
Sun Ray Institute answered |
Sulphuric acid is a compound that contains sulphur in its formula, represented by the symbol "S" in H2SO4.
Sulphur dioxide (SO2) is a product of which process?- a)Photosynthesis
- b)Respiration
- c)Combustion of fossil fuels
- d)Fermentation
Correct answer is option 'C'. Can you explain this answer?
Sulphur dioxide (SO2) is a product of which process?
a)
Photosynthesis
b)
Respiration
c)
Combustion of fossil fuels
d)
Fermentation

|
Sun Ray Institute answered |
Sulphur dioxide is released during the combustion of fossil fuels, such as coal and oil, which contain sulphur impurities.
What is the primary application of sulfur in the petroleum industry?- a)Fuel production
- b)Lubricant manufacturing
- c)Explosive synthesis
- d)Desulfurization processes
Correct answer is option 'D'. Can you explain this answer?
What is the primary application of sulfur in the petroleum industry?
a)
Fuel production
b)
Lubricant manufacturing
c)
Explosive synthesis
d)
Desulfurization processes

|
Sun Ray Institute answered |
Sulfur is used in desulfurization processes in the petroleum industry to remove sulfur compounds from fuels. This helps reduce the emission of harmful sulfur oxides during combustion.
Which allotrope of sulfur is commonly known as "flowers of sulfur"?- a)Rhombic sulfur
- b)Monoclinic sulfur
- c)Amorphous sulfur
- d)Precious sulfur
Correct answer is option 'A'. Can you explain this answer?
Which allotrope of sulfur is commonly known as "flowers of sulfur"?
a)
Rhombic sulfur
b)
Monoclinic sulfur
c)
Amorphous sulfur
d)
Precious sulfur

|
Sun Ray Institute answered |
Rhombic sulfur is commonly referred to as "flowers of sulfur." It is a yellow crystalline solid that resembles tiny flowers or powder. Rhombic sulfur is one of the most stable allotropes of sulfur.
Chapter doubts & questions for Sulphur - Chemistry for Grade 9 2025 is part of Grade 9 exam preparation. The chapters have been prepared according to the Grade 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Sulphur - Chemistry for Grade 9 in English & Hindi are available as part of Grade 9 exam.
Download more important topics, notes, lectures and mock test series for Grade 9 Exam by signing up for free.
Chemistry for Grade 9
63 videos|123 docs|66 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily

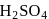 and
and 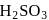 can be distinguished by the addition of
can be distinguished by the addition of solution
solution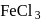 solution
solution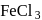 acts as oxidant and
acts as oxidant and 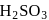 as
as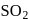 is formed?
is formed?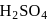 with
with 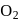
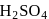
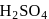 with
with 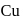
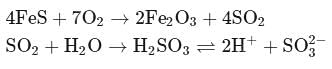
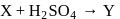 (a colourless and irritating gas)
(a colourless and irritating gas)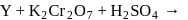 (green coloured solution)
(green coloured solution)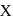 and
and 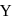 .
.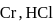
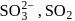
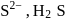
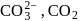
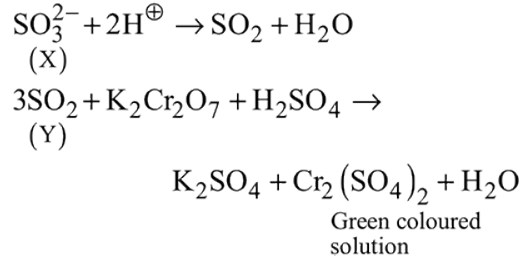
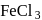 solution on reaction with
solution on reaction with 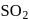 changes to
changes to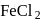
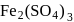
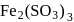
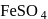
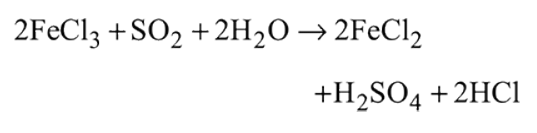
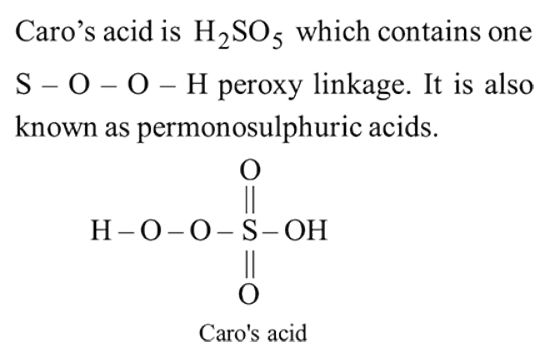
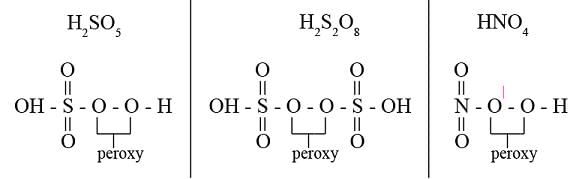
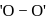 peroxy bond is present in :-
peroxy bond is present in :-