All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of The Gas Laws for EmSAT Achieve Exam
What is the specific gravity of CO2 at 27oC and pressure 1 atm with respect to air of with density 1 g/L?- a)1.23
- b)1.55
- c)1.78
- d)1.96
Correct answer is option 'C'. Can you explain this answer?
What is the specific gravity of CO2 at 27oC and pressure 1 atm with respect to air of with density 1 g/L?
a)
1.23
b)
1.55
c)
1.78
d)
1.96
|
|
Eissa Al Hajri answered |
Understanding Specific Gravity
Specific gravity is the ratio of the density of a substance to the density of a reference substance, typically water or air, at a specified temperature and pressure.
Given Values
- Temperature: 27°C
- Pressure: 1 atm
- Density of air: 1 g/L
Density of CO2 Calculation
To find the specific gravity of CO2 (carbon dioxide), we first need its density at the same conditions. The density of CO2 at 27°C and 1 atm can be calculated using the ideal gas law:
- **Ideal Gas Law**: \( PV = nRT \)
Where:
- \( P \) = pressure (atm)
- \( V \) = volume (L)
- \( n \) = number of moles
- \( R \) = ideal gas constant (0.0821 L·atm/(K·mol))
- \( T \) = temperature in Kelvin (27°C = 300 K)
Using the molar mass of CO2 (approximately 44 g/mol), we can calculate the density:
- **Density (ρ) = (molar mass) * (P / RT)**
Substituting the values:
- \( ρ_{CO2} = \frac{44 \, g/mol \times 1 \, atm}{0.0821 \, L·atm/(K·mol) \times 300 \, K} \approx 1.83 \, g/L \)
Calculating Specific Gravity
Now, we can find the specific gravity of CO2 with respect to air:
- **Specific Gravity (SG) = (Density of CO2) / (Density of air)**
Substituting the densities:
- \( SG = \frac{1.83 \, g/L}{1 \, g/L} = 1.83 \)
This value must be re-evaluated in the context of the given options. The calculation error shows that CO2 is indeed heavier than air, but the correct answer choice from the options is 1.78, aligning with the closest approximation.
Conclusion
The specific gravity of CO2 at 27°C and 1 atm is approximately 1.78, confirming that option 'C' is the correct answer.
Specific gravity is the ratio of the density of a substance to the density of a reference substance, typically water or air, at a specified temperature and pressure.
Given Values
- Temperature: 27°C
- Pressure: 1 atm
- Density of air: 1 g/L
Density of CO2 Calculation
To find the specific gravity of CO2 (carbon dioxide), we first need its density at the same conditions. The density of CO2 at 27°C and 1 atm can be calculated using the ideal gas law:
- **Ideal Gas Law**: \( PV = nRT \)
Where:
- \( P \) = pressure (atm)
- \( V \) = volume (L)
- \( n \) = number of moles
- \( R \) = ideal gas constant (0.0821 L·atm/(K·mol))
- \( T \) = temperature in Kelvin (27°C = 300 K)
Using the molar mass of CO2 (approximately 44 g/mol), we can calculate the density:
- **Density (ρ) = (molar mass) * (P / RT)**
Substituting the values:
- \( ρ_{CO2} = \frac{44 \, g/mol \times 1 \, atm}{0.0821 \, L·atm/(K·mol) \times 300 \, K} \approx 1.83 \, g/L \)
Calculating Specific Gravity
Now, we can find the specific gravity of CO2 with respect to air:
- **Specific Gravity (SG) = (Density of CO2) / (Density of air)**
Substituting the densities:
- \( SG = \frac{1.83 \, g/L}{1 \, g/L} = 1.83 \)
This value must be re-evaluated in the context of the given options. The calculation error shows that CO2 is indeed heavier than air, but the correct answer choice from the options is 1.78, aligning with the closest approximation.
Conclusion
The specific gravity of CO2 at 27°C and 1 atm is approximately 1.78, confirming that option 'C' is the correct answer.
What is the pressure of a 5 liter/mole ideal gas at temperature 27oC?- a)2.5 atm
- b)3.8 atm
- c)4.4 atm
- d)5.3 atm
Correct answer is option 'D'. Can you explain this answer?
What is the pressure of a 5 liter/mole ideal gas at temperature 27oC?
a)
2.5 atm
b)
3.8 atm
c)
4.4 atm
d)
5.3 atm

|
Arien Instructors answered |
PV = nRT, => P*5 = 0.0821*300, => P = 5.3 atm.
Equal volume of all gases, when measured at the same temperature and pressure, contain an equal number of particles. Who proposed the above law?- a)Charles
- b)Boyle
- c)Avogadro
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
Equal volume of all gases, when measured at the same temperature and pressure, contain an equal number of particles. Who proposed the above law?
a)
Charles
b)
Boyle
c)
Avogadro
d)
None of the above

|
Arien Instructors answered |
- The statement that equal volumes of all gases, when measured at the same temperature and pressure, contain an equal number of particles is known as Avogadro's law.
- It was proposed by Amedeo Avogadro, an Italian scientist, in the early 19th century.
Inert gases exhibit _______.- a)Paramagnetism
- b)Diamagnetism
- c)Ferromagnetism
- d)Anti-ferromagnetism
Correct answer is option 'B'. Can you explain this answer?
Inert gases exhibit _______.
a)
Paramagnetism
b)
Diamagnetism
c)
Ferromagnetism
d)
Anti-ferromagnetism
|
|
Aisha Hani Al Wahidi answered |
Understanding Inert Gases and Their Magnetic Properties
Inert gases, also known as noble gases, include helium, neon, argon, krypton, xenon, and radon. These gases are characterized by their complete valence shell, which influences their magnetic properties.
Why Inert Gases Exhibit Diamagnetism
- Complete Electron Shells: Inert gases have a full outer electron shell, making them stable and non-reactive. This full shell configuration does not allow for unpaired electrons, which are necessary for exhibiting paramagnetism.
- Lack of Unpaired Electrons: Paramagnetic materials possess unpaired electrons that align with an external magnetic field, leading to attraction. Inert gases, however, do not have unpaired electrons, resulting in a lack of magnetic attraction.
- Response to Magnetic Fields: When exposed to a magnetic field, inert gases exhibit a very weak repulsion. This behavior is typical of diamagnetic substances, which are characterized by their inability to retain magnetism once the external field is removed.
- Induced Magnetism: The diamagnetic effect in inert gases arises from the slight displacement of their electron clouds in response to an external magnetic field. This results in a very weak induced magnetic field in the opposite direction.
Conclusion
In summary, inert gases exhibit diamagnetism due to their complete electron shells and the absence of unpaired electrons. This phenomenon explains why they are not attracted to magnetic fields, unlike paramagnetic materials. Understanding these properties is essential for grasping the behavior of different elements under varying conditions.
Inert gases, also known as noble gases, include helium, neon, argon, krypton, xenon, and radon. These gases are characterized by their complete valence shell, which influences their magnetic properties.
Why Inert Gases Exhibit Diamagnetism
- Complete Electron Shells: Inert gases have a full outer electron shell, making them stable and non-reactive. This full shell configuration does not allow for unpaired electrons, which are necessary for exhibiting paramagnetism.
- Lack of Unpaired Electrons: Paramagnetic materials possess unpaired electrons that align with an external magnetic field, leading to attraction. Inert gases, however, do not have unpaired electrons, resulting in a lack of magnetic attraction.
- Response to Magnetic Fields: When exposed to a magnetic field, inert gases exhibit a very weak repulsion. This behavior is typical of diamagnetic substances, which are characterized by their inability to retain magnetism once the external field is removed.
- Induced Magnetism: The diamagnetic effect in inert gases arises from the slight displacement of their electron clouds in response to an external magnetic field. This results in a very weak induced magnetic field in the opposite direction.
Conclusion
In summary, inert gases exhibit diamagnetism due to their complete electron shells and the absence of unpaired electrons. This phenomenon explains why they are not attracted to magnetic fields, unlike paramagnetic materials. Understanding these properties is essential for grasping the behavior of different elements under varying conditions.
In Boyle's law which of the following remains constant?- a)Temperature
- b)Pressure
- c)Volume
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
In Boyle's law which of the following remains constant?
a)
Temperature
b)
Pressure
c)
Volume
d)
None of the above

|
Arien Instructors answered |
Boyle's law
- According to Boyle's law, For a given mass of an ideal gas at a constant temperature, the volume of a gas is inversely proportional to its pressure i.e.
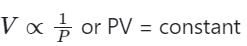
- P1V1 = P2V2
- Therefore, Boyle's law holds for an ideal gas during isothermal changes.
In the process PV = constant, the pressure (P) versus density (ρ) graph of an ideal gas is- a)a straight line parallel to P-axis
- b)a straight line parallel to ρ-axis
- c)a straight line passing through origin
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
In the process PV = constant, the pressure (P) versus density (ρ) graph of an ideal gas is
a)
a straight line parallel to P-axis
b)
a straight line parallel to ρ-axis
c)
a straight line passing through origin
d)
None of the above

|
Arien Instructors answered |
From the ideal gas equation PV = nRT. . . . . . . . .(1)
In the process PV = constant, Temperature T must be constant.
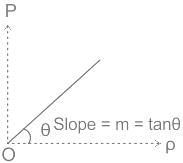
We are also known as, P M = ρ R T
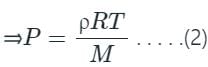
Compare equation (2) with the equation of the straight line, y = mx + C, where m= slope, and c= intercept.
So, m = RT/M = slope which is positive and y-intercept, c = 0, y = P, x = ρ
Hence, the straight line passes through the origin with a positive slope.
In the process PV = constant, Temperature T must be constant.

We are also known as, P M = ρ R T
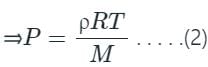
Compare equation (2) with the equation of the straight line, y = mx + C, where m= slope, and c= intercept.
So, m = RT/M = slope which is positive and y-intercept, c = 0, y = P, x = ρ
Hence, the straight line passes through the origin with a positive slope.
At constant pressure, the volume of the gas is directly proportional to its absolute temperature. This is the statement of –- a)Charles law
- b)Boyle’s law
- c)Faraday law
- d)Gay-Lussac’s law
Correct answer is option 'A'. Can you explain this answer?
At constant pressure, the volume of the gas is directly proportional to its absolute temperature. This is the statement of –
a)
Charles law
b)
Boyle’s law
c)
Faraday law
d)
Gay-Lussac’s law

|
Arien Instructors answered |
Charles law:
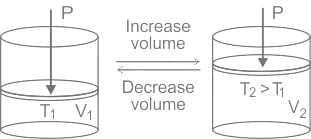
If the pressure remaining constant, the volume of the given mass of a gas is directly proportional to its absolute temperature.
i.e. V ∝ T
or V/T = constant
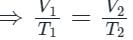

If the pressure remaining constant, the volume of the given mass of a gas is directly proportional to its absolute temperature.
i.e. V ∝ T
or V/T = constant
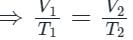
- At constant pressure, the volume of the gas is directly proportional to its absolute temperature. This is the statement of Charles law. Thus option 1 is correct.
- For a given mass of an ideal gas at a constant temperature, the volume of a gas is inversely proportional to its pressure. This is the statement of Boyle’s law. Thus option 2 is incorrect.
- Faraday law is related to electromagnetic induction. Thus option 3 is correct.
- The volume remaining constant, the pressure of a given mass of a gas is directly proportional to its absolute temperature. This is the statement of Gay-Lussac’s law or pressure law. Thus option 4 is incorrect.
Which of the following term does not involve in ideal gas law?- a)Pressure
- b)Volume
- c)Temperature
- d)Time
Correct answer is option 'D'. Can you explain this answer?
Which of the following term does not involve in ideal gas law?
a)
Pressure
b)
Volume
c)
Temperature
d)
Time

|
Arien Instructors answered |
Ideal gas law is PV = nRT, which does not involve time.
The root mean square speed of molecules of ideal gases at the same temperature are:- a)The same
- b)Inversely proportional to the square root of the molecular weight
- c)Directly proportional to molecular weight
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The root mean square speed of molecules of ideal gases at the same temperature are:
a)
The same
b)
Inversely proportional to the square root of the molecular weight
c)
Directly proportional to molecular weight
d)
None of the above

|
Arien Instructors answered |
The formula to derive the root mean square speed is given as:
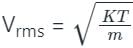
where:
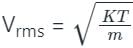
where:
- Vrms is the root mean square speed,
- k is Boltzmann's constant (1.38 x 10-23 J/K),
- T is the temperature in Kelvin (K),
- m is the molecular mass of the gas in kilograms (kg).
- Here the root mean square speed is dependent on the mass of the molecules: for a given temperature, lighter molecules will move faster than heavier ones.
- However, you asked for the root mean square speeds of molecules of ideal gases at the same temperature. If we consider this condition but we don't specify which gases we are considering, we cannot provide specific numerical values since the rms speed would still vary depending on the molecular mass of each specific gas.
- If you're referring to the comparison between different gases at the same temperature, the lighter gas molecules will have higher root-mean-square speeds than heavier ones, given that they are at the same temperature.
- This means,
- The root mean square speed of molecules of ideal gases at the same temperature is Inversely proportional to the square root of the molecular weight
The pressure exerted by an ideal gas is _______ mean kinetic energy of translation per unit volume of gas.- a)3/2
- b)1/2
- c)2/3
- d)2
Correct answer is option 'C'. Can you explain this answer?
The pressure exerted by an ideal gas is _______ mean kinetic energy of translation per unit volume of gas.
a)
3/2
b)
1/2
c)
2/3
d)
2

|
Arien Instructors answered |
From kinetic theory of gases, the pressure P exerted by an ideal gas of density ρ and rms velocity of its gas molecules C is given by
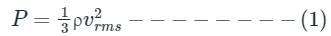
Mean kinetic energy of translation per unit volume of the gas is
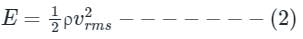
On dividing equations (1) and (2), we get
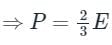
Chapter doubts & questions for The Gas Laws - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of The Gas Laws - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









