All Exams >
MCAT >
General Chemistry for MCAT >
All Questions
All questions of Thermodynamics for MCAT Exam
Additional gas is pumped inside a rigid container that stores compressed gas. Which of the following is a true statement about this system?- a)Pressure is constant throughout the compression.
- b)There is no work done on the container.
- c)The molar concentration of gas is decreasing.
- d)The volume of the container is decreasing.
Correct answer is option 'B'. Can you explain this answer?
Additional gas is pumped inside a rigid container that stores compressed gas. Which of the following is a true statement about this system?
a)
Pressure is constant throughout the compression.
b)
There is no work done on the container.
c)
The molar concentration of gas is decreasing.
d)
The volume of the container is decreasing.
|
|
Samuel Lewis answered |
Explanation:
When additional gas is pumped inside a rigid container that stores compressed gas, several changes occur in the system. Let's analyze each statement to determine which one is true.
a) Pressure is constant throughout the compression:
This statement is not true. When additional gas is pumped into the container, the volume of the container remains constant while the number of gas molecules increases. According to the ideal gas law (PV = nRT), if the volume (V) is constant and the number of moles (n) increases, then the pressure (P) must also increase. Therefore, the pressure is not constant throughout the compression.
b) There is no work done on the container:
This statement is true. Since the container is rigid, there is no change in volume, and work is defined as the product of force and displacement. Without a change in volume, there is no displacement, and therefore no work is done on the container.
c) The molar concentration of gas is decreasing:
This statement is not true. When additional gas is pumped into the container, the number of gas molecules increases, while the volume remains constant. As a result, the molar concentration of the gas, which is defined as the number of moles of gas divided by the volume, will increase rather than decrease.
d) The volume of the container is decreasing:
This statement is not true. The problem states that additional gas is pumped into the container, which implies that the volume of the container is not decreasing. In a rigid container, the volume remains constant regardless of the changes in the amount of gas inside.
Therefore, the correct statement is option 'B' - There is no work done on the container.
When additional gas is pumped inside a rigid container that stores compressed gas, several changes occur in the system. Let's analyze each statement to determine which one is true.
a) Pressure is constant throughout the compression:
This statement is not true. When additional gas is pumped into the container, the volume of the container remains constant while the number of gas molecules increases. According to the ideal gas law (PV = nRT), if the volume (V) is constant and the number of moles (n) increases, then the pressure (P) must also increase. Therefore, the pressure is not constant throughout the compression.
b) There is no work done on the container:
This statement is true. Since the container is rigid, there is no change in volume, and work is defined as the product of force and displacement. Without a change in volume, there is no displacement, and therefore no work is done on the container.
c) The molar concentration of gas is decreasing:
This statement is not true. When additional gas is pumped into the container, the number of gas molecules increases, while the volume remains constant. As a result, the molar concentration of the gas, which is defined as the number of moles of gas divided by the volume, will increase rather than decrease.
d) The volume of the container is decreasing:
This statement is not true. The problem states that additional gas is pumped into the container, which implies that the volume of the container is not decreasing. In a rigid container, the volume remains constant regardless of the changes in the amount of gas inside.
Therefore, the correct statement is option 'B' - There is no work done on the container.
When heating a solution, a scientist detects a temperature increase in the solution during a period of time. Which of the following statements accurately characterizes the solution during this period?- a)The solution is at boiling point.
- b)The solution is undergoing a phase change.
- c)The velocity of molecules in the solution is increasing.
- d)The solution’s temperature increase is proportional to its ΔHvaporization
Correct answer is option 'C'. Can you explain this answer?
When heating a solution, a scientist detects a temperature increase in the solution during a period of time. Which of the following statements accurately characterizes the solution during this period?
a)
The solution is at boiling point.
b)
The solution is undergoing a phase change.
c)
The velocity of molecules in the solution is increasing.
d)
The solution’s temperature increase is proportional to its ΔHvaporization
|
|
Julian Gray answered |
Is experiencing a chemical reaction.
Which of the following scenarios violates the first law of thermodynamics, “the conservation of energy?"- a)A spring that extends and retracts forever, alternating between potential and kinetic energy.
- b)An isolated electrochemical cell that indefinitely generates an electrical current.
- c)An efficient wind turbine that converts all of its energy from mechanical movement into electrical potential energy.
- d)A machine that converts heat energy into work energy.
Correct answer is option 'B'. Can you explain this answer?
Which of the following scenarios violates the first law of thermodynamics, “the conservation of energy?"
a)
A spring that extends and retracts forever, alternating between potential and kinetic energy.
b)
An isolated electrochemical cell that indefinitely generates an electrical current.
c)
An efficient wind turbine that converts all of its energy from mechanical movement into electrical potential energy.
d)
A machine that converts heat energy into work energy.
|
|
Emma Smith answered |
The first law of thermodynamics states that energy cannot be created or destroyed, only transferred or converted from one form to another. Therefore, any scenario that involves the creation or destruction of energy would violate the first law of thermodynamics.
1. A car engine converts chemical energy from gasoline into thermal energy and mechanical energy to move the car. This does not violate the first law of thermodynamics as energy is being converted from one form to another.
2. A refrigerator uses electrical energy to transfer heat from inside the refrigerator to the outside, cooling the contents inside. This also does not violate the first law of thermodynamics as energy is being transferred from one location to another.
3. A perpetual motion machine that creates energy out of nothing and can operate indefinitely without an external energy source. This scenario violates the first law of thermodynamics as it involves the creation of energy from nothing, which is not possible according to the law.
4. A power plant that produces electricity by burning fossil fuels and releases waste heat into the environment. This does not violate the first law of thermodynamics as energy is being converted from chemical energy in the fuel to electrical energy, and the waste heat is transferred to the environment.
5. A solar panel that converts sunlight into electrical energy. This also does not violate the first law of thermodynamics as energy is being converted from solar radiation to electrical energy.
1. A car engine converts chemical energy from gasoline into thermal energy and mechanical energy to move the car. This does not violate the first law of thermodynamics as energy is being converted from one form to another.
2. A refrigerator uses electrical energy to transfer heat from inside the refrigerator to the outside, cooling the contents inside. This also does not violate the first law of thermodynamics as energy is being transferred from one location to another.
3. A perpetual motion machine that creates energy out of nothing and can operate indefinitely without an external energy source. This scenario violates the first law of thermodynamics as it involves the creation of energy from nothing, which is not possible according to the law.
4. A power plant that produces electricity by burning fossil fuels and releases waste heat into the environment. This does not violate the first law of thermodynamics as energy is being converted from chemical energy in the fuel to electrical energy, and the waste heat is transferred to the environment.
5. A solar panel that converts sunlight into electrical energy. This also does not violate the first law of thermodynamics as energy is being converted from solar radiation to electrical energy.
A hot object is placed next to a cold object so that they are touching. Which of the following statements is true?
I. Heat will transfer from the hot object to the cold object because the hot object has a higher temperature.
II. The two objects are in thermal equilibrium
III. Internal energy will transfer from the hot object to the cold object because the hot object has greater internal energy.- a)I
- b)II
- c)I & III
- d)III
Correct answer is option 'A'. Can you explain this answer?
A hot object is placed next to a cold object so that they are touching. Which of the following statements is true?
I. Heat will transfer from the hot object to the cold object because the hot object has a higher temperature.
II. The two objects are in thermal equilibrium
III. Internal energy will transfer from the hot object to the cold object because the hot object has greater internal energy.
I. Heat will transfer from the hot object to the cold object because the hot object has a higher temperature.
II. The two objects are in thermal equilibrium
III. Internal energy will transfer from the hot object to the cold object because the hot object has greater internal energy.
a)
I
b)
II
c)
I & III
d)
III
|
|
Emma Smith answered |
C) I
What is the net work done on the gas as it goes from point C to D and then to E on the Pressure vs Volume diagram?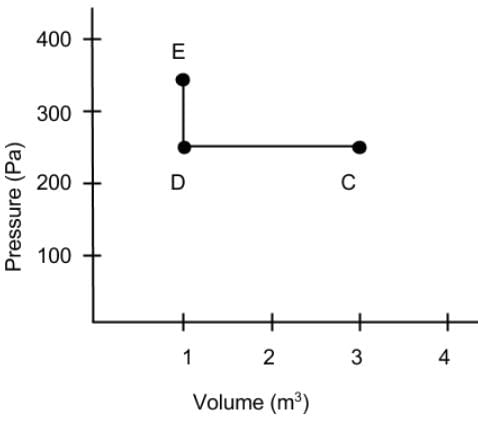
- a)0 J
- b)-500 J
- c)500 J
- d)1000 J
Correct answer is option 'C'. Can you explain this answer?
What is the net work done on the gas as it goes from point C to D and then to E on the Pressure vs Volume diagram?

a)
0 J
b)
-500 J
c)
500 J
d)
1000 J
|
|
Ayesha Joshi answered |
We can calculate work as the area under the line graph in the diagram.
The volume of the gas is decreased, so the value of work done on the gas is positive.
The magnitude of work will be the area under the function from C to D. The path from D to E is vertical, so there is no work being done during this portion.
250 Pa x 2 m3 = 500 J.
And 500 J + 0J = 500 J.
And 500 J + 0J = 500 J.
What is the net work done on the gas as it goes from point A to B on the Pressure vs Volume diagram?
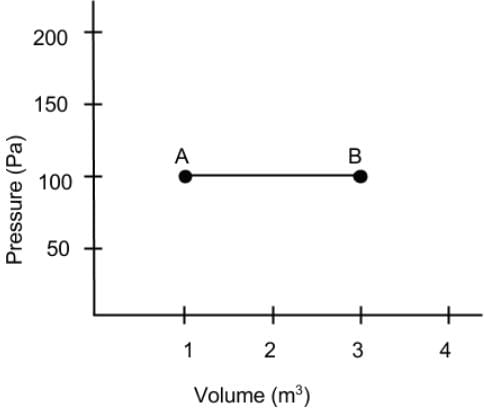
- a)200 J
- b)0 J
- c)-200 J
- d)100 J
Correct answer is option 'C'. Can you explain this answer?
What is the net work done on the gas as it goes from point A to B on the Pressure vs Volume diagram?


a)
200 J
b)
0 J
c)
-200 J
d)
100 J

|
Orion Classes answered |
Since the gas is kept at a constant pressure, we can calculate work with W = PΔV.
The volume of the gas is increased so the value of work done on the gas is negative.
The magnitude of work will be the area of under the function from A to B. Which is 200 in the case. The net work done on the gas as it goes from point A to B is −200 J.
Equal amounts of heat are absorbed by 100 g, samples of various solid metals with differing specific heat values. Which of the following statements is true regarding metals and their specific heat values?- a)The metal with the smallest specific heat will undergo the smallest change in temperature.
- b)The metal with the smallest specific heat will resist melting to a greater degree at its melting point.
- c)The metal with the greatest specific heat will undergo the smallest change in temperature.
- d)The metal with the greatest specific heat will resist melting to a greater degree at its melting point.
Correct answer is option 'C'. Can you explain this answer?
Equal amounts of heat are absorbed by 100 g, samples of various solid metals with differing specific heat values. Which of the following statements is true regarding metals and their specific heat values?
a)
The metal with the smallest specific heat will undergo the smallest change in temperature.
b)
The metal with the smallest specific heat will resist melting to a greater degree at its melting point.
c)
The metal with the greatest specific heat will undergo the smallest change in temperature.
d)
The metal with the greatest specific heat will resist melting to a greater degree at its melting point.
|
|
Ayesha Joshi answered |
The specific heat capacity refers to the amount of heat required to cause a unit of mass to change its temperature by 1°C.
The specific heat capacity does not refer to a material’s resistance to melting at its melting point. This is value is referred to as the heat of fusion, ΔHfusion
The specific heat capacity is a proportion that affects how a material’s absorption or release of heat changes its temperature. It is detailed by the equation: q = mcΔT, where q is heat, c is the specific heat capacity, and ΔT is the change in temperature.
According to the q = mcΔT equation, as long as the provided masses of metal solids are the same, the metal with the greatest specific heat will undergo the smallest change in temperature for a given heat energy value.
Atmospheric gases absorb more energy than they emit. If we consider a gas to be a closed system, which of the following is true?- a)The heat absorbed by the gas is positive.
- b)The internal energy of the gas increases.
- c)The change in volume of the gas is negative.
- d)The work done on the gas is equal to the change in internal energy and the heat absorbed by the gas.
Correct answer is option 'A'. Can you explain this answer?
Atmospheric gases absorb more energy than they emit. If we consider a gas to be a closed system, which of the following is true?
a)
The heat absorbed by the gas is positive.
b)
The internal energy of the gas increases.
c)
The change in volume of the gas is negative.
d)
The work done on the gas is equal to the change in internal energy and the heat absorbed by the gas.
|
|
Ayesha Joshi answered |
According to the given statement, atmospheric gases absorb more energy than they emit. In thermodynamics, heat is defined as the transfer of energy between a system and its surroundings due to a temperature difference. When a gas absorbs heat, it means that energy is entering the gas from its surroundings, resulting in an increase in the internal energy of the gas. Therefore, the heat absorbed by the gas is positive.
In a system undergoing adiabatic compression, what are the values of internal energy and heat if work done on the system is 500 J?- a)Internal energy is 0 J and heat is 500 J.
- b)Internal energy is -500 J and heat is 0 J.
- c)Internal energy is 0 J and heat is -500 J.
- d)Internal energy is 500 J and heat is 0 J.
Correct answer is option 'D'. Can you explain this answer?
In a system undergoing adiabatic compression, what are the values of internal energy and heat if work done on the system is 500 J?
a)
Internal energy is 0 J and heat is 500 J.
b)
Internal energy is -500 J and heat is 0 J.
c)
Internal energy is 0 J and heat is -500 J.
d)
Internal energy is 500 J and heat is 0 J.
|
|
Ayesha Joshi answered |
This is an adiabatic process which means there is no heat transfer, q = 0.
According to the first law of thermodynamics, internal energy is equal to the sum of the heat within the system and the work done on the system. ΔU = q + w
Following the first law of thermodynamics equation:
According to the first law of thermodynamics, internal energy is equal to the sum of the heat within the system and the work done on the system. ΔU = q + w
Following the first law of thermodynamics equation:
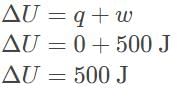
Which of the following best defines enthalpy?- a)The measure of disorder in a system
- b)The amount of heat released or absorbed in a chemical reaction
- c)The energy required to break chemical bonds
- d)The capacity to do work or transfer heat
Correct answer is option 'B'. Can you explain this answer?
Which of the following best defines enthalpy?
a)
The measure of disorder in a system
b)
The amount of heat released or absorbed in a chemical reaction
c)
The energy required to break chemical bonds
d)
The capacity to do work or transfer heat
|
|
Ayesha Joshi answered |
An exothermic reaction is a chemical reaction that releases heat energy to the surroundings. It is characterized by a negative change in enthalpy, indicating a decrease in the energy content of the system.
Chapter doubts & questions for Thermodynamics - General Chemistry for MCAT 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermodynamics - General Chemistry for MCAT in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
General Chemistry for MCAT
164 videos|11 docs|16 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









