All Exams >
MCAT >
General Chemistry for MCAT >
All Questions
All questions of Acid/base Equilibria for MCAT Exam
Which of the following best describes a chemical species that is measured to have Kb = 3.2 x 10-18?- a)A weak base
- b)A strong base
- c)A strong acid
- d)A weak acid
Correct answer is option 'C'. Can you explain this answer?
Which of the following best describes a chemical species that is measured to have Kb = 3.2 x 10-18?
a)
A weak base
b)
A strong base
c)
A strong acid
d)
A weak acid
|
|
Harper White answered |
Understanding Kb Values
The Kb value measures the strength of a base in solution. A small Kb indicates that the base does not ionize significantly in water, suggesting it is weak.
Analyzing Kb = 3.2 x 10^-18
- The given Kb value of 3.2 x 10^-18 is extremely low.
- This means that the base has very limited ability to accept protons (H+ ions) in aqueous solution.
- Consequently, this low Kb value indicates that it is not an effective base.
Identifying the Chemical Species
- A weak base, like the one described, is characterized by its inability to fully dissociate in solution.
- This contrasts sharply with strong bases, which possess high Kb values and fully dissociate in water.
Why Option 'C' is Incorrect
- The assertion that the chemical species is a strong acid contradicts the low Kb value.
- Strong acids have high Ka values (acid dissociation constants), while weak acids have low Ka values.
- The Kb value provided does not align with the characteristics of a strong acid or weak acid.
Conclusion
- The correct classification of a chemical species with Kb = 3.2 x 10^-18 is a weak base.
- Understanding Kb and its implications is crucial in categorizing acid-base behavior in chemistry.
In summary, always remember: a low Kb indicates a weak base, not a strong acid.
The Kb value measures the strength of a base in solution. A small Kb indicates that the base does not ionize significantly in water, suggesting it is weak.
Analyzing Kb = 3.2 x 10^-18
- The given Kb value of 3.2 x 10^-18 is extremely low.
- This means that the base has very limited ability to accept protons (H+ ions) in aqueous solution.
- Consequently, this low Kb value indicates that it is not an effective base.
Identifying the Chemical Species
- A weak base, like the one described, is characterized by its inability to fully dissociate in solution.
- This contrasts sharply with strong bases, which possess high Kb values and fully dissociate in water.
Why Option 'C' is Incorrect
- The assertion that the chemical species is a strong acid contradicts the low Kb value.
- Strong acids have high Ka values (acid dissociation constants), while weak acids have low Ka values.
- The Kb value provided does not align with the characteristics of a strong acid or weak acid.
Conclusion
- The correct classification of a chemical species with Kb = 3.2 x 10^-18 is a weak base.
- Understanding Kb and its implications is crucial in categorizing acid-base behavior in chemistry.
In summary, always remember: a low Kb indicates a weak base, not a strong acid.
Which of the following would have the weakest conjugate acid?- a)A strong base
- b)A weak acid
- c)A strong acid
- d)A weak base
Correct answer is option 'A'. Can you explain this answer?
Which of the following would have the weakest conjugate acid?
a)
A strong base
b)
A weak acid
c)
A strong acid
d)
A weak base
|
|
Lucas Anderson answered |
The strength of a conjugate acid refers to its ability to donate a proton (H+) in a chemical reaction. The weaker the conjugate acid, the less likely it is to donate a proton. In this case, we are comparing the strength of conjugate acids based on the given options: a strong base, a weak acid, a strong acid, and a weak base.
To determine the weakest conjugate acid, we need to consider the strength of the corresponding base. The stronger the base, the weaker its conjugate acid will be.
Explanation:
a) A strong base:
A strong base is a substance that completely dissociates in water to produce hydroxide ions (OH-). Examples of strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH). Since strong bases completely dissociate, they have a high concentration of hydroxide ions. As a result, their conjugate acids will be weak because hydroxide ions readily accept protons, making it less likely for them to donate a proton. Therefore, the weakest conjugate acid is associated with a strong base.
b) A weak acid:
A weak acid is a substance that only partially dissociates in water to produce hydrogen ions (H+). Examples of weak acids include acetic acid (CH3COOH) and formic acid (HCOOH). Weak acids have a limited ability to donate protons, so their conjugate acids will be stronger compared to a strong base.
c) A strong acid:
A strong acid is a substance that completely dissociates in water to produce hydrogen ions (H+). Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4). Strong acids have a high concentration of hydrogen ions, so their conjugate acids will be weak.
d) A weak base:
A weak base is a substance that only partially accepts protons or donates electrons. Examples of weak bases include ammonia (NH3) and water (H2O). Weak bases have a limited ability to accept protons, so their conjugate acids will be stronger compared to a strong base.
Conclusion:
Based on the explanations above, the weakest conjugate acid is associated with a strong base (option A). Strong bases have a high concentration of hydroxide ions, making it less likely for them to donate protons and resulting in weaker conjugate acids.
To determine the weakest conjugate acid, we need to consider the strength of the corresponding base. The stronger the base, the weaker its conjugate acid will be.
Explanation:
a) A strong base:
A strong base is a substance that completely dissociates in water to produce hydroxide ions (OH-). Examples of strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH). Since strong bases completely dissociate, they have a high concentration of hydroxide ions. As a result, their conjugate acids will be weak because hydroxide ions readily accept protons, making it less likely for them to donate a proton. Therefore, the weakest conjugate acid is associated with a strong base.
b) A weak acid:
A weak acid is a substance that only partially dissociates in water to produce hydrogen ions (H+). Examples of weak acids include acetic acid (CH3COOH) and formic acid (HCOOH). Weak acids have a limited ability to donate protons, so their conjugate acids will be stronger compared to a strong base.
c) A strong acid:
A strong acid is a substance that completely dissociates in water to produce hydrogen ions (H+). Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4). Strong acids have a high concentration of hydrogen ions, so their conjugate acids will be weak.
d) A weak base:
A weak base is a substance that only partially accepts protons or donates electrons. Examples of weak bases include ammonia (NH3) and water (H2O). Weak bases have a limited ability to accept protons, so their conjugate acids will be stronger compared to a strong base.
Conclusion:
Based on the explanations above, the weakest conjugate acid is associated with a strong base (option A). Strong bases have a high concentration of hydroxide ions, making it less likely for them to donate protons and resulting in weaker conjugate acids.
Suppose a large organic molecule X is classified as a Lewis acid, while another large molecule Y is classified as a Bronsted-Lowry acid. Which of the following most accurately describes a similarity in their behaviors in solution?- a)Both molecules will tend to acquire a net positive charge
- b)Both molecules will release hydroxide ions
- c)Both molecules will tend to acquire a net negative charge
- d)Both molecules will release hydrogen gas
Correct answer is option 'C'. Can you explain this answer?
Suppose a large organic molecule X is classified as a Lewis acid, while another large molecule Y is classified as a Bronsted-Lowry acid. Which of the following most accurately describes a similarity in their behaviors in solution?
a)
Both molecules will tend to acquire a net positive charge
b)
Both molecules will release hydroxide ions
c)
Both molecules will tend to acquire a net negative charge
d)
Both molecules will release hydrogen gas
|
|
Ayesha Joshi answered |
A Bronsted-Lowry acid tends to donate hydrogen ions, which have positive charges in solution.
This leaves the rest of the molecule with a net negative charge.
A Lewis acid tends to accept electrons in solution, leaving the molecule with a net negative charge
Thus both X and Y will tend to acquire a net negative charge in solution
Which of the following describes the pH of an equilibrated, stoichiometric mixture of ammonia, NH3 and hydrochloric acid HCl- a)pH > 7
- b)pH ≈ 7
- c)pH < 7
- d)pH = 7
Correct answer is option 'C'. Can you explain this answer?
Which of the following describes the pH of an equilibrated, stoichiometric mixture of ammonia, NH3 and hydrochloric acid HCl
a)
pH > 7
b)
pH ≈ 7
c)
pH < 7
d)
pH = 7
|
|
Ayesha Joshi answered |
Ammonia is a weak base
Hydrochloric acid is a strong acid
The hydrochloric acid will donate a proton to the ammonia to form ammonium,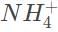
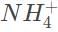
Ammonium reacts with water to form hydronium,
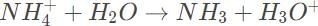
A mixture of a strong acid and a weak base acquires an overall acidic pH < 7
Suppose a weak acid has Kα = 4.0 x 10-9. Which of the following gives its equivalent Kb ?- a)2.5 x 10-6
- b)1.5 x 10-10
- c)4.0 x 10-11
- d)4.0 x 10-8
Correct answer is option 'A'. Can you explain this answer?
Suppose a weak acid has Kα = 4.0 x 10-9. Which of the following gives its equivalent Kb ?
a)
2.5 x 10-6
b)
1.5 x 10-10
c)
4.0 x 10-11
d)
4.0 x 10-8
|
|
Ayesha Joshi answered |
Recall that an acid has both Kα and a Kb the relative values of which depend on the nature of its interaction with water.
Recall that Kw = 10-14
Recall KαKb = Kw, and so Kb = 2.5 x 10-6
What is the pH of a solution with a hydronium ion concentration[H3O+] = 104 M- a)-8
- b)-4
- c)10
- d)4
Correct answer is option 'B'. Can you explain this answer?
What is the pH of a solution with a hydronium ion concentration
[H3O+] = 104 M
a)
-8
b)
-4
c)
10
d)
4
|
|
Ayesha Joshi answered |
pH is the negative logarithm of the molar concentration of hydrogen ions
Equivalently, the pH is the logarithm of the molar concentration of hydronium
pH = - log[H3O+] = -4
Suppose an equilibrated, dilute solution containing an acid H A with Kα = 10-4 is measured to have pH = 6 and [HA] = 10-8 M. Which of the following gives the best estimate of [A-]- a)10-4 M
- b)10-6 M
- c)10-14 M
- d)10-12 M
Correct answer is option 'B'. Can you explain this answer?
Suppose an equilibrated, dilute solution containing an acid H A with Kα = 10-4 is measured to have pH = 6 and [HA] = 10-8 M. Which of the following gives the best estimate of [A-]
a)
10-4 M
b)
10-6 M
c)
10-14 M
d)
10-12 M
|
|
Ayesha Joshi answered |
Recall the Henderson-Hasselbach equation, which is valid for the dilute solution described here, 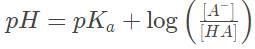
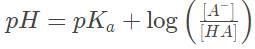
Recall that pKα =−log Kα = 4
Solving the Henderson-Hasselbach equation yields [A-] = 10-6 M
Suppose a nanotechnological innovation allows every single charged ion to be precisely identified and removed from a small volume of water. Which of the following describes Kα for the water at the end of the process, assuming that the filtered water is given adequate time to re-equilibrate?- a)10-14
- b)10-7
- c)1
- d)0
Correct answer is option 'A'. Can you explain this answer?
Suppose a nanotechnological innovation allows every single charged ion to be precisely identified and removed from a small volume of water. Which of the following describes Kα for the water at the end of the process, assuming that the filtered water is given adequate time to re-equilibrate?
a)
10-14
b)
10-7
c)
1
d)
0
|
|
Ayesha Joshi answered |
The autoionization of water occurs naturally due to attractive forces between constituents of water molecules.
Even if all ions are removed from a sample of water (including hydronium ions), a short time later the water will re-ionize until it approaches an equilibrium concentration of ions.
In equilibrium, Kα = Kw = 10-14
Hypochlorous acid dissociates in water to create hydronium ions and hypochlorite ions HOCl + H2O ⇔ H3O+ + OCl- Suppose that additional hypochlorite ions are added to the solution. Which of the following correctly describes the resultant effect on the concentration of HOCl?- a)It depends on the number of hydronium ions
- b)It remains the same
- c)It increases
- d)It decreases
Correct answer is option 'C'. Can you explain this answer?
Hypochlorous acid dissociates in water to create hydronium ions and hypochlorite ions HOCl + H2O ⇔ H3O+ + OCl- Suppose that additional hypochlorite ions are added to the solution. Which of the following correctly describes the resultant effect on the concentration of HOCl?
a)
It depends on the number of hydronium ions
b)
It remains the same
c)
It increases
d)
It decreases
|
|
Ayesha Joshi answered |
Le Châtelier's principle states that equilibrated solutions tend to resist changes in the relative concentrations of chemical species.
The reaction thus seeks to minimize the effect of the added OCl- ions, tilting the equilibrium to the left.
The concentration of HOCl increases.
Suppose an acid H A has a dissociation constant Kα = 1 x 10-1. It is mixed into a buffered solution, and its equilibrium concentration is [H A] = .1M. If the concentration of its conjugate base is 10 M, what is the pH of the solution?- a)4
- b)3
- c)8
- d)1
Correct answer is option 'B'. Can you explain this answer?
Suppose an acid H A has a dissociation constant Kα = 1 x 10-1. It is mixed into a buffered solution, and its equilibrium concentration is [H A] = .1M. If the concentration of its conjugate base is 10 M, what is the pH of the solution?
a)
4
b)
3
c)
8
d)
1
|
|
Ayesha Joshi answered |
Recall the Henderson-Hasselbach equation, which is valid for the buffer solution described here, 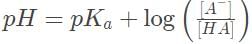
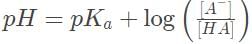
Recall that pKα = - log Kα = 1
Now solve the other term,
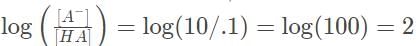
The pH is thus pH = 1 + 2 = 3
Chapter doubts & questions for Acid/base Equilibria - General Chemistry for MCAT 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Acid/base Equilibria - General Chemistry for MCAT in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
General Chemistry for MCAT
164 videos|11 docs|16 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









