All Exams >
MCAT >
General Chemistry for MCAT >
All Questions
All questions of Chemical Kinetics for MCAT Exam
Which of the following is an example of a zero-order reaction?- a)Radioactive decay
- b)First-order decay
- c)Second-order reaction
- d)Photochemical reaction
Correct answer is option 'A'. Can you explain this answer?
Which of the following is an example of a zero-order reaction?
a)
Radioactive decay
b)
First-order decay
c)
Second-order reaction
d)
Photochemical reaction
|
|
Harper White answered |
Zero-Order Reaction
A zero-order reaction is a type of chemical reaction in which the rate of the reaction is independent of the concentrations of the reactants. This means that the reaction proceeds at a constant rate, regardless of how much reactant is present. The rate equation for a zero-order reaction is given by:
Rate = k
where k is the rate constant.
Radioactive Decay
Radioactive decay is an example of a zero-order reaction. It is the process by which unstable atomic nuclei spontaneously break apart, releasing radiation and transforming into a different element. The rate of radioactive decay is determined solely by the decay constant, which is a characteristic property of the radioactive material.
Explanation
In a zero-order reaction, the rate of the reaction does not depend on the concentration of the reactants. This means that even if the concentration of the reactant is doubled or halved, the rate of the reaction will remain the same.
Radioactive decay follows this behavior because it is a first-order reaction with respect to the radioactive material. The rate of decay is determined solely by the decay constant, which is a property of the specific radioactive isotope. The decay constant represents the probability of a radioactive atom decaying per unit time and is independent of the concentration of the radioactive material.
For example, if we have a sample of radioactive material with an initial concentration of 1 mol/L, the rate of decay will be a constant value determined by the decay constant. If we double the concentration of the radioactive material to 2 mol/L, the rate of decay will still be the same constant value. Similarly, if we halve the concentration to 0.5 mol/L, the rate of decay will remain unchanged.
Conclusion
Radioactive decay is an example of a zero-order reaction because the rate of decay is independent of the concentration of the radioactive material. The rate of decay is determined solely by the decay constant, which is a characteristic property of the radioactive isotope.
A zero-order reaction is a type of chemical reaction in which the rate of the reaction is independent of the concentrations of the reactants. This means that the reaction proceeds at a constant rate, regardless of how much reactant is present. The rate equation for a zero-order reaction is given by:
Rate = k
where k is the rate constant.
Radioactive Decay
Radioactive decay is an example of a zero-order reaction. It is the process by which unstable atomic nuclei spontaneously break apart, releasing radiation and transforming into a different element. The rate of radioactive decay is determined solely by the decay constant, which is a characteristic property of the radioactive material.
Explanation
In a zero-order reaction, the rate of the reaction does not depend on the concentration of the reactants. This means that even if the concentration of the reactant is doubled or halved, the rate of the reaction will remain the same.
Radioactive decay follows this behavior because it is a first-order reaction with respect to the radioactive material. The rate of decay is determined solely by the decay constant, which is a property of the specific radioactive isotope. The decay constant represents the probability of a radioactive atom decaying per unit time and is independent of the concentration of the radioactive material.
For example, if we have a sample of radioactive material with an initial concentration of 1 mol/L, the rate of decay will be a constant value determined by the decay constant. If we double the concentration of the radioactive material to 2 mol/L, the rate of decay will still be the same constant value. Similarly, if we halve the concentration to 0.5 mol/L, the rate of decay will remain unchanged.
Conclusion
Radioactive decay is an example of a zero-order reaction because the rate of decay is independent of the concentration of the radioactive material. The rate of decay is determined solely by the decay constant, which is a characteristic property of the radioactive isotope.
Which of the following steps in this multi-step chemical reaction will be the rate determining step?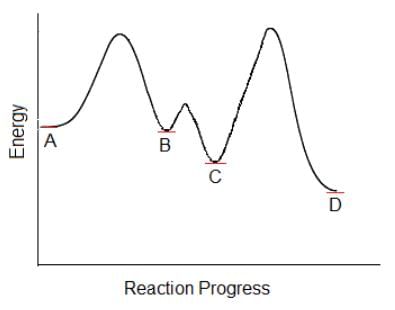
- a)B→C
- b)C→B
- c)A→B
- d)C→D
Correct answer is option 'D'. Can you explain this answer?
Which of the following steps in this multi-step chemical reaction will be the rate determining step?

a)
B→C
b)
C→B
c)
A→B
d)
C→D

|
Orion Classes answered |
The rate determining step is the slowest step of a reaction.
The reaction with the slowest rate will have the highest activation energy, or largest energy difference between the reactant and transition state.
The step between C and D has the largest difference of energy between reactant (B) and the transition state and therefore this step has highest activation energy. This is the rate-determining step.
In the following reaction coordinate diagram, each point represents a state a molecule passes through as it undergoes the reaction. Which of the following could be isolated during the course of the reaction?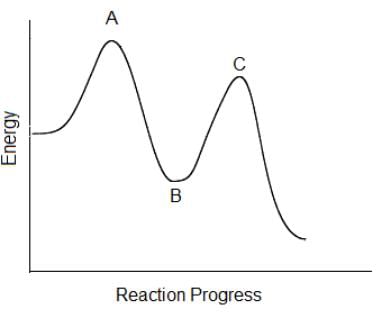
- a)A
- b)B
- c)C
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
In the following reaction coordinate diagram, each point represents a state a molecule passes through as it undergoes the reaction. Which of the following could be isolated during the course of the reaction?

a)
A
b)
B
c)
C
d)
None of the above
|
|
Ayesha Joshi answered |
This question is testing the difference between an intermediate and a transition state. An intermediate differs from a transition state in that the intermediate has a discrete lifetime, whereas a transition state is simply the transition to a new molecule, and is then the higher points on the reaction coordinate.
A transition state (both points A+C) is the transition to a new molecule, it is simply a very high energy state that cannot be isolated during a reaction.
An intermediate is a short-lived unstable molecule in a reaction. It shows a slight reduction in energy on the reaction coordinate (point B), and can be isolated during a reaction.
Which of the following statements is true regarding activation energy?- a)It is the energy required for a reaction to proceed.
- b)It determines the overall energy change of a reaction.
- c)It remains constant for all chemical reactions.
- d)It depends on the concentration of reactants.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements is true regarding activation energy?
a)
It is the energy required for a reaction to proceed.
b)
It determines the overall energy change of a reaction.
c)
It remains constant for all chemical reactions.
d)
It depends on the concentration of reactants.
|
|
Ayesha Joshi answered |
Activation energy is the minimum amount of energy required for a chemical reaction to occur. It represents the energy barrier that reactant molecules must overcome to transform into products. Activation energy is specific to each reaction and depends on the nature of the reactants and the reaction pathway.
The Arrhenius equation, k = Ae-Ea/(RT) gives the relationship of the rate constant of a reaction to the temperature (T) and the activation energy (Ea). If a catalyst is added that decreases the activation energy by 20 kJ/mol, and simultaneously the temperature is decreased by 20 K, which of the following will be true of the reaction?- a)The reaction rate will decrease overall
- b)Not enough information is given
- c)The reaction rate will increase overall
- d)The reaction will take place at the same rate
Correct answer is option 'C'. Can you explain this answer?
The Arrhenius equation, k = Ae-Ea/(RT) gives the relationship of the rate constant of a reaction to the temperature (T) and the activation energy (Ea). If a catalyst is added that decreases the activation energy by 20 kJ/mol, and simultaneously the temperature is decreased by 20 K, which of the following will be true of the reaction?
a)
The reaction rate will decrease overall
b)
Not enough information is given
c)
The reaction rate will increase overall
d)
The reaction will take place at the same rate
|
|
Mia Anderson answered |
The Arrhenius Equation:
The Arrhenius equation, k = Ae^(-Ea/RT), relates the rate constant (k) of a reaction to the temperature (T) and the activation energy (Ea). The rate constant is a measure of how fast a reaction proceeds. The equation shows that as the temperature increases or the activation energy decreases, the rate constant increases, indicating a faster reaction rate.
Effect of a Catalyst:
A catalyst is a substance that increases the rate of a chemical reaction by providing an alternative reaction pathway with a lower activation energy. In the case of this question, a catalyst is added that decreases the activation energy by 20 kJ/mol. This means that the value of Ea in the Arrhenius equation would be decreased by 20 kJ/mol when the catalyst is present.
Effect of Decreasing Temperature:
Simultaneously, the temperature is decreased by 20 K. The temperature (T) in the Arrhenius equation is in Kelvin, and decreasing the temperature by 20 K would result in a lower value for T.
Effect on the Reaction Rate:
The combination of adding a catalyst that decreases the activation energy and decreasing the temperature has two opposing effects on the reaction rate:
1. Effect of the Catalyst:
By decreasing the activation energy, the catalyst provides an alternative reaction pathway that requires less energy for the reactants to reach the transition state. This leads to an increase in the rate constant and, consequently, an increase in the reaction rate.
2. Effect of Decreasing Temperature:
Decreasing the temperature lowers the value of T in the Arrhenius equation. Since T is in the denominator of the equation, a lower T value would result in a smaller rate constant and a slower reaction rate.
Overall Effect:
Since the temperature and activation energy are both decreased by 20 units, their opposing effects on the reaction rate can be canceled out to some extent. However, since the activation energy is decreased by the catalyst, the overall effect is an increase in the rate constant and an increase in the reaction rate. Therefore, the correct answer is option C: The reaction rate will increase overall.
The Arrhenius equation, k = Ae^(-Ea/RT), relates the rate constant (k) of a reaction to the temperature (T) and the activation energy (Ea). The rate constant is a measure of how fast a reaction proceeds. The equation shows that as the temperature increases or the activation energy decreases, the rate constant increases, indicating a faster reaction rate.
Effect of a Catalyst:
A catalyst is a substance that increases the rate of a chemical reaction by providing an alternative reaction pathway with a lower activation energy. In the case of this question, a catalyst is added that decreases the activation energy by 20 kJ/mol. This means that the value of Ea in the Arrhenius equation would be decreased by 20 kJ/mol when the catalyst is present.
Effect of Decreasing Temperature:
Simultaneously, the temperature is decreased by 20 K. The temperature (T) in the Arrhenius equation is in Kelvin, and decreasing the temperature by 20 K would result in a lower value for T.
Effect on the Reaction Rate:
The combination of adding a catalyst that decreases the activation energy and decreasing the temperature has two opposing effects on the reaction rate:
1. Effect of the Catalyst:
By decreasing the activation energy, the catalyst provides an alternative reaction pathway that requires less energy for the reactants to reach the transition state. This leads to an increase in the rate constant and, consequently, an increase in the reaction rate.
2. Effect of Decreasing Temperature:
Decreasing the temperature lowers the value of T in the Arrhenius equation. Since T is in the denominator of the equation, a lower T value would result in a smaller rate constant and a slower reaction rate.
Overall Effect:
Since the temperature and activation energy are both decreased by 20 units, their opposing effects on the reaction rate can be canceled out to some extent. However, since the activation energy is decreased by the catalyst, the overall effect is an increase in the rate constant and an increase in the reaction rate. Therefore, the correct answer is option C: The reaction rate will increase overall.
Which of the following are true of reaction rates?
I. The overall rate law is determined by the fastest step of a reaction
II. The presence of a catalyst will increase the number of molecules entering the transition state
III. An increase in temperature will increase the rate of a reaction
IV. Increasing the concentration of reactants will increase the rate at which products yield- a)I+II+IV
- b)I +II+III
- c)II+III only
- d)II+III+IV
Correct answer is option 'C'. Can you explain this answer?
Which of the following are true of reaction rates?
I. The overall rate law is determined by the fastest step of a reaction
II. The presence of a catalyst will increase the number of molecules entering the transition state
III. An increase in temperature will increase the rate of a reaction
IV. Increasing the concentration of reactants will increase the rate at which products yield
I. The overall rate law is determined by the fastest step of a reaction
II. The presence of a catalyst will increase the number of molecules entering the transition state
III. An increase in temperature will increase the rate of a reaction
IV. Increasing the concentration of reactants will increase the rate at which products yield
a)
I+II+IV
b)
I +II+III
c)
II+III only
d)
II+III+IV
|
|
Ayesha Joshi answered |
The overall rate law is determined by the slowest step of a reaction, not the fastest.
The higher the temperature of a reaction, the faster the reaction rate.
Normally, the greater the concentration of reactants, the faster the reaction rate will occur. However this is not true of zero order reactions! Based on what you know about zero order reactions, see if you can convince yourself of this!
A catalyst will decrease the activation energy, and any decrease in activation energy will increase the reaction rate, therefore II and III are correct.
Given the following information, what would the overall order of the reaction be?
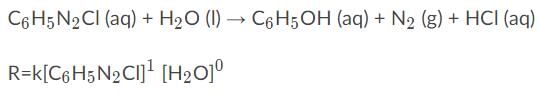
- a)0
- b)1
- c)2
- d)3
Correct answer is option 'B'. Can you explain this answer?
Given the following information, what would the overall order of the reaction be?
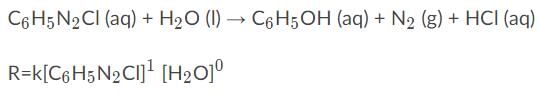
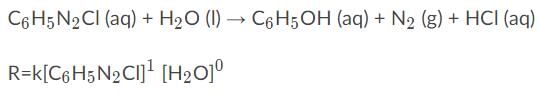
a)
0
b)
1
c)
2
d)
3
|
|
Radha Iyer answered |
The overall order of a reaction is the sum of the individual reaction orders.
The following reaction is first order for C6H5N2Cl and zero order for H2O
To find the overall order you would add the order of C6H5N2Cl and H2O, or 1+0 = 1.
Which of the following factors would increase the rate of a gaseous reaction?- a)Decreasing the temperature
- b)Increasing the volume
- c)Decreasing the pressure
- d)Adding an inert gas
Correct answer is option 'B'. Can you explain this answer?
Which of the following factors would increase the rate of a gaseous reaction?
a)
Decreasing the temperature
b)
Increasing the volume
c)
Decreasing the pressure
d)
Adding an inert gas
|
|
Ayesha Joshi answered |
Increasing the volume of a gaseous reaction system would decrease the pressure. According to Le Chatelier's principle, the system would shift towards the side with more moles of gas to alleviate the pressure change. This would increase the rate of the reaction if there are more moles of gaseous reactants than products.
Which of the following is true regarding the addition of a catalyst to a reaction?- a)The rate of the reverse reaction is decreased
- b)The ΔG of a reaction is decreased
- c)The equilibrium favors the products upon catalyst addition
- d)The energy of the activated complex will decrease
Correct answer is option 'D'. Can you explain this answer?
Which of the following is true regarding the addition of a catalyst to a reaction?
a)
The rate of the reverse reaction is decreased
b)
The ΔG of a reaction is decreased
c)
The equilibrium favors the products upon catalyst addition
d)
The energy of the activated complex will decrease
|
|
Ayesha Joshi answered |
A catalyst does not affect the thermodynamics of a reaction, only the kinetics. Therefore ΔG will not change.
The addition of a catalyst will increase the rate of both the forward and reverse reactions.
The equilibrium of a reaction is not changed upon the addition of the catalyst, only the rate at which the reaction reaches equilibrium is changed.
‘Activated’ complex is an alternate term for the reactants in transition state.
The activated complex is formed upon reaching the required activation energy level. This required energy is what is decreased upon the addition of a catalyst.
Chapter doubts & questions for Chemical Kinetics - General Chemistry for MCAT 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Kinetics - General Chemistry for MCAT in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
General Chemistry for MCAT
164 videos|11 docs|16 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









