All Exams >
MCAT >
Physics for MCAT >
All Questions
All questions of Gas Phase for MCAT Exam
Assuming ideal gas properties, which of the following occupies the most volume at 273 K and 1 atm of pressure?- a)One mole of hydrogen gas
- b)They all occupy the same volume
- c)One mole of oxygen gas
- d)One mole of nitrogen gas
Correct answer is option 'B'. Can you explain this answer?
Assuming ideal gas properties, which of the following occupies the most volume at 273 K and 1 atm of pressure?
a)
One mole of hydrogen gas
b)
They all occupy the same volume
c)
One mole of oxygen gas
d)
One mole of nitrogen gas
|
|
Grace Adams answered |
Understanding Ideal Gas Behavior
At standard temperature and pressure (STP)—273 K and 1 atm—ideal gas behavior can be analyzed using the Ideal Gas Law. The law states that:
PV = nRT
Where:
- P = Pressure
- V = Volume
- n = Number of moles
- R = Universal gas constant
- T = Temperature
Volume Occupied by Gases
According to the Ideal Gas Law, the volume occupied by a gas is directly proportional to the number of moles when temperature and pressure are constant. Therefore, one mole of any ideal gas occupies the same volume under identical conditions of temperature and pressure.
Calculation at STP
- At STP, 1 mole of an ideal gas occupies approximately 22.4 liters.
- This is true for any ideal gas, regardless of its molecular weight or type (H2, O2, N2, etc.).
Conclusion
Thus, the correct answer to the question is:
- They all occupy the same volume.
This means that:
- One mole of hydrogen gas occupies 22.4 liters.
- One mole of oxygen gas occupies 22.4 liters.
- One mole of nitrogen gas occupies 22.4 liters.
Since they all have the same number of moles (1 mole), and the conditions are the same (273 K and 1 atm), all gases will occupy the same volume, confirming that option "B" is correct.
At standard temperature and pressure (STP)—273 K and 1 atm—ideal gas behavior can be analyzed using the Ideal Gas Law. The law states that:
PV = nRT
Where:
- P = Pressure
- V = Volume
- n = Number of moles
- R = Universal gas constant
- T = Temperature
Volume Occupied by Gases
According to the Ideal Gas Law, the volume occupied by a gas is directly proportional to the number of moles when temperature and pressure are constant. Therefore, one mole of any ideal gas occupies the same volume under identical conditions of temperature and pressure.
Calculation at STP
- At STP, 1 mole of an ideal gas occupies approximately 22.4 liters.
- This is true for any ideal gas, regardless of its molecular weight or type (H2, O2, N2, etc.).
Conclusion
Thus, the correct answer to the question is:
- They all occupy the same volume.
This means that:
- One mole of hydrogen gas occupies 22.4 liters.
- One mole of oxygen gas occupies 22.4 liters.
- One mole of nitrogen gas occupies 22.4 liters.
Since they all have the same number of moles (1 mole), and the conditions are the same (273 K and 1 atm), all gases will occupy the same volume, confirming that option "B" is correct.
How many diagrams showing relationships between pressure, volume, and temperature below is/are incorrect for ideal gases?
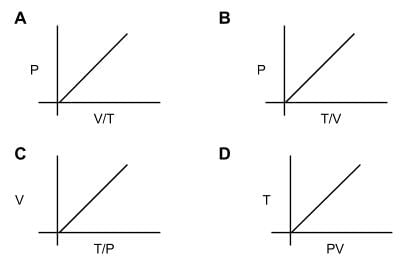
- a)Only one
- b)Only three
- c)Only two
- d)None
Correct answer is option 'A'. Can you explain this answer?
How many diagrams showing relationships between pressure, volume, and temperature below is/are incorrect for ideal gases?


a)
Only one
b)
Only three
c)
Only two
d)
None

|
Orion Classes answered |
- Although the graphs may look daunting, all of the relationships can be derived using the ideal gas law, PV=nRT.
- Just rearrange the variables in the gas law so we can see if there is a positive correlation (on different sides of the equal sign) or a negative correlation (on the same side).
- For A: P⋅V/T = nR, so this will be a negative correlation. A is false. For B: P = nR⋅T/V. B is true. For C: V = nR⋅T/P. C is true. For D: PV = nR⋅T. D is true.
- Only one diagram shows an incorrect relationship for ideal gases.
Assume an experiment vessel which is well fitted with a piston. The airtight piston has negligible mass and can move up and down freely. There is 1 mol of an ideal gas contained within the vessel under the piston at pressure of 100 kPa. The piston rests at 10 cm from the base of the vessel. The pressure in the vessel increases to 200 kPa as force is applied to the piston. What would be the new resting position of the piston from the base of the vessel? Assume the temperature is held constant at 300 K throughout the experiment.- a)5 cm
- b)2 cm
- c)1 cm
- d)8 cm
Correct answer is option 'A'. Can you explain this answer?
Assume an experiment vessel which is well fitted with a piston. The airtight piston has negligible mass and can move up and down freely. There is 1 mol of an ideal gas contained within the vessel under the piston at pressure of 100 kPa. The piston rests at 10 cm from the base of the vessel. The pressure in the vessel increases to 200 kPa as force is applied to the piston. What would be the new resting position of the piston from the base of the vessel? Assume the temperature is held constant at 300 K throughout the experiment.
a)
5 cm
b)
2 cm
c)
1 cm
d)
8 cm
|
|
Ayesha Joshi answered |
- Try using Boyle's Law for this question.
- P1V1 = P2V2 , then V2 = P1V1/P2. It's important to realize that to find the volume occupied by the gas in this experiment vessel, we would use A (area of the piston) multiplied by h (the height of the piston). We then would have Ah2 = P1Ah1/P2. The surface areas actually cancel out, and you will have h2 = P1h1/P2.
- Substituting in the values, you will get the new resting position as 5 cm.
Shown below are four mercury barometers of the same height (all four barometer tubes measure one meter from the tube opening to rounded top). Which barometer shows the greatest external pressure?
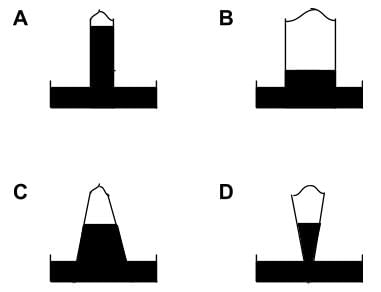
- a)Barometer C
- b)Barometer D
- c)Barometer A
- d)Barometer B
Correct answer is option 'C'. Can you explain this answer?
Shown below are four mercury barometers of the same height (all four barometer tubes measure one meter from the tube opening to rounded top). Which barometer shows the greatest external pressure?


a)
Barometer C
b)
Barometer D
c)
Barometer A
d)
Barometer B
|
|
Ayesha Joshi answered |
- Consider the difference in shapes between the different barometers.
- Given that the force acting on the fluid in the barometer is F = mg. We can derive it thus: m = density⋅volume, so F = density⋅volume⋅g. Volume = area⋅height, so F=density⋅area⋅height⋅g. We know that F/area = pressure, so we can say that the change in pressure = the change in height⋅density⋅g.
Atmospheric air is comprised, roughly, of 80% nitrogen and 20% oxygen. A 100 L sample of atmospheric air is kept at 300 K and 100 kPa. How many moles of oxygen molecules are found in this gas sample? (Use R = 10)
(L⋅kPa)/(mol⋅K))- a)4/5
- b)2/3
- c)3/2
- d)1/5
Correct answer is option 'B'. Can you explain this answer?
Atmospheric air is comprised, roughly, of 80% nitrogen and 20% oxygen. A 100 L sample of atmospheric air is kept at 300 K and 100 kPa. How many moles of oxygen molecules are found in this gas sample? (Use R = 10)
(L⋅kPa)/(mol⋅K))
(L⋅kPa)/(mol⋅K))
a)
4/5
b)
2/3
c)
3/2
d)
1/5
|
|
Ayesha Joshi answered |
- The law of partial pressures states that the volume of each gas in the mixture is proportional to the partial pressures of each gas in the mixture.
- Given atmospheric air is 20% oxygen, the partial pressure of oxygen in this sample is 20 kPa. Now use the ideal gas equation to find n.
- n = PV/RT,orn = 100⋅20/10⋅300 = 2/3moles.
Gases found in the environment are most likely to exhibit properties similar to that of ideal gases under conditions of:- a)low temperatures and high pressures
- b)high temperatures and high pressures
- c)high temperatures and low pressures
- d)low temperatures and low pressures
Correct answer is option 'C'. Can you explain this answer?
Gases found in the environment are most likely to exhibit properties similar to that of ideal gases under conditions of:
a)
low temperatures and high pressures
b)
high temperatures and high pressures
c)
high temperatures and low pressures
d)
low temperatures and low pressures
|
|
Ayesha Joshi answered |
- Think back to the assumptions of the ideal gas laws.
- In order to obey the ideal gas laws, we would need to minimize the effects of intermolecular interactions and molecular size of these gases. Thus, the ideal conditions will be under high temperatures (lots of kinetic energy in the molecules to move around and not be experiencing forces from each other) and low pressure (less inhibited to move around).
- Gases found in the environment are most likely to exhibit properties similar to that of ideal gases under conditions of high temperatures and low pressures.
Which properties reflected in real gases does the van der Waals equation attempt to account for by modifying the ideal gas law?
I. Volume
II. Pressure
III. Temperature- a)II
- b)I and II
- c)I, II and III
- d)I
Correct answer is option 'B'. Can you explain this answer?
Which properties reflected in real gases does the van der Waals equation attempt to account for by modifying the ideal gas law?
I. Volume
II. Pressure
III. Temperature
I. Volume
II. Pressure
III. Temperature
a)
II
b)
I and II
c)
I, II and III
d)
I
|
|
Ayesha Joshi answered |
- Real gases experience intermolecular forces between gas molecules (van der Waals forces).
- Real gases molecules possess mass.
- Given that real gases experience intermolecular forces and possess mass, the van der Waals equation attempts to modify the ideal gas law by introducing the constants a and b, which adjusts for the effects of volume and pressure in real gases. (Real gases have lower pressure and higher volume compared to ideal gases) There is no term in the van der Waals equation that adjusts for temperature.
All of the following are properties of ideal gases except:- a)Small amounts of energy are lost during collisions between gas molecules
- b)Volume occupied by molecules is negligible compared to the volume occupied by the gas
- c)Collisions between gas molecules are completely elastic
- d)Gas molecules do not interact with each other except during collisions
Correct answer is option 'A'. Can you explain this answer?
All of the following are properties of ideal gases except:
a)
Small amounts of energy are lost during collisions between gas molecules
b)
Volume occupied by molecules is negligible compared to the volume occupied by the gas
c)
Collisions between gas molecules are completely elastic
d)
Gas molecules do not interact with each other except during collisions
|
|
Ayesha Joshi answered |
- The property listed in option 1, "Small amounts of energy are lost during collisions between gas molecules," is not a characteristic of ideal gases. In reality, energy can be lost or gained during collisions between gas molecules. Ideal gases are a theoretical concept that assumes certain simplifying assumptions to make calculations and predictions easier.
- One of these assumptions is that gas molecules collide elastically, as stated in option 3. This means that during collisions, there is no loss of kinetic energy, and the total energy of the gas remains constant. However, in real gases, some energy can be transferred in the form of heat or converted into other forms, leading to a loss of energy.
- In an ideal gas, the molecules are considered to have negligible volume compared to the total volume occupied by the gas, as mentioned in option 2. This assumption allows us to ignore the individual volumes of gas molecules and treat the gas as a collection of point particles.
- Option 4 states that gas molecules do not interact with each other except during collisions. This assumption implies that there are no attractive or repulsive forces between gas molecules. In reality, intermolecular forces can exist between gas molecules, although they may be weak in some cases.
- In summary, option 1 is not a property of ideal gases because ideal gases assume completely elastic collisions, while in reality, some energy can be lost or gained during collisions between gas molecules.
The behavior of which of these real gases will be reflected most closely with the ideal gas law?- a)CO2
- b)Ar
- c)CH4
- d)He
Correct answer is option 'D'. Can you explain this answer?
The behavior of which of these real gases will be reflected most closely with the ideal gas law?
a)
CO2
b)
Ar
c)
CH4
d)
He
|
|
Ayesha Joshi answered |
- Consider the factors that distinguish real gases from ideal gases (i.e. what the van der Waals equation attempts to take into account)
- Large gas molecules generally possess more volume and intermolecular forces.
- Carbon dioxide and methane are large molecules, which immediately means more mass in each molecule, causing deviations from ideal gas behavior. Between helium and argon (both monomolecular noble gases), helium is the smaller gas molecule of the two, which similarly will have less of a mass effect, demonstrating closer adherence to the ideal gas law.
At a given temperature T and pressure P, a person's lung holds a set volume of V of air. Given that air is roughly 20% oxygen, how many moles of oxygen are in his lungs?- a)0.2*(PT/RV)
- b)5*(PT/RV)
- c)0.2*(PV/RT)
- d)5*(PV/RT)
Correct answer is option 'C'. Can you explain this answer?
At a given temperature T and pressure P, a person's lung holds a set volume of V of air. Given that air is roughly 20% oxygen, how many moles of oxygen are in his lungs?
a)
0.2*(PT/RV)
b)
5*(PT/RV)
c)
0.2*(PV/RT)
d)
5*(PV/RT)
|
|
Ayesha Joshi answered |
- Use the ideal gas law to find n (moles of air).
- n = PV/RT. This includes a mixture of nitrogen, oxygen, and other gases. From the mole fraction rule, we can infer that if the air is 20% oxygen, 20% of the total moles must be oxygen molecules.
- Thus, there are 0.2*(PV/RT) moles of oxygen in his lungs.
Chapter doubts & questions for Gas Phase - Physics for MCAT 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Gas Phase - Physics for MCAT in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
Physics for MCAT
158 videos|21 docs|21 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









