All Exams >
JEE >
JEE Main & Advanced Mock Test Series 2025 >
All Questions
All questions of JEE Main Mock Test series 2026 for JEE Exam
At a certain temperature in a 5 L vessel, 2 moles of carbon monoxide and 3 moles of chlorine were allowed to reach equilibrium, according to the following reaction:
CO + Cl2 ⇌ COCl2
At equilibrium, if 1 mole of CO is present, then equilibrium constant (KC) for the reaction is:(Nearest integer)Correct answer is '3'. Can you explain this answer?
At a certain temperature in a 5 L vessel, 2 moles of carbon monoxide and 3 moles of chlorine were allowed to reach equilibrium, according to the following reaction:
CO + Cl2 ⇌ COCl2
At equilibrium, if 1 mole of CO is present, then equilibrium constant (KC) for the reaction is:(Nearest integer)
CO + Cl2 ⇌ COCl2
At equilibrium, if 1 mole of CO is present, then equilibrium constant (KC) for the reaction is:(Nearest integer)

|
Tarun Kaushik answered |
Initially, 2 moles of CO are present.
At equilibrium, 1 mole of CO is present.
Hence, 2 - 1 = 1 mole of CO has reacted.
1 mole of CO will react with 1 mole of Cl2 to form 1 mole of COCl2.
3 - 1 = 2 moles of Cl2 remains at equilibrium.
The equilibrium constant
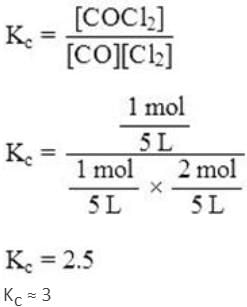
At equilibrium, 1 mole of CO is present.
Hence, 2 - 1 = 1 mole of CO has reacted.
1 mole of CO will react with 1 mole of Cl2 to form 1 mole of COCl2.
3 - 1 = 2 moles of Cl2 remains at equilibrium.
The equilibrium constant
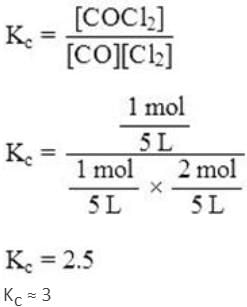
If the tangents drawn to the hyperbola 4y2 = x2 + 1 intersect the co-ordinate axes at distinct points A and B, then the locus of the midpoint of AB is- a)4x2 - y2 + 16x2y2 = 0
- b)x2 - 4y2 + 16x2y2 = 0
- c)x2 - 4y2 - 16x2y2 = 0
- d)4x2 - y2 - 16x2y2 = 0
Correct answer is option 'C'. Can you explain this answer?
If the tangents drawn to the hyperbola 4y2 = x2 + 1 intersect the co-ordinate axes at distinct points A and B, then the locus of the midpoint of AB is
a)
4x2 - y2 + 16x2y2 = 0
b)
x2 - 4y2 + 16x2y2 = 0
c)
x2 - 4y2 - 16x2y2 = 0
d)
4x2 - y2 - 16x2y2 = 0
|
|
Shalini Dasgupta answered |
Understanding the Hyperbola
The given hyperbola is represented by the equation:
4y² = x² + 1
This can be rewritten in the standard form for hyperbolas.
Equation of the Tangents
The general equation of the tangent to the hyperbola at a point (x₁, y₁) is derived from the hyperbola's equation. For the hyperbola, the tangent can be expressed as:
y = mx ± √(a²m² + b²)
Here, m is the slope of the tangent and (a, b) are constants derived from the hyperbola's equation.
Finding Intersection Points A and B
The tangents intersect the coordinate axes at points A (x₁, 0) and B (0, y₁). By substituting y = 0 in the tangent equation, we get the x-coordinate, and substituting x = 0 provides the y-coordinate.
Midpoint of AB
The midpoint M of A and B can be expressed as:
M = ((x₁ + 0)/2, (0 + y₁)/2) = (x₁/2, y₁/2)
Deriving the Locus of M
To find the locus, we express x₁ and y₁ in terms of m, leading to the equation relating x and y coordinates. After manipulation, the locus can be derived as follows:
x² - 4y² - 16x²y² = 0
This represents the required locus of the midpoint of points A and B.
Conclusion
The correct option for the locus of the midpoint of AB is:
c) x² - 4y² - 16x²y² = 0
This indicates that the locus is a specific curve defined by the equation derived from the tangents to the hyperbola.
The given hyperbola is represented by the equation:
4y² = x² + 1
This can be rewritten in the standard form for hyperbolas.
Equation of the Tangents
The general equation of the tangent to the hyperbola at a point (x₁, y₁) is derived from the hyperbola's equation. For the hyperbola, the tangent can be expressed as:
y = mx ± √(a²m² + b²)
Here, m is the slope of the tangent and (a, b) are constants derived from the hyperbola's equation.
Finding Intersection Points A and B
The tangents intersect the coordinate axes at points A (x₁, 0) and B (0, y₁). By substituting y = 0 in the tangent equation, we get the x-coordinate, and substituting x = 0 provides the y-coordinate.
Midpoint of AB
The midpoint M of A and B can be expressed as:
M = ((x₁ + 0)/2, (0 + y₁)/2) = (x₁/2, y₁/2)
Deriving the Locus of M
To find the locus, we express x₁ and y₁ in terms of m, leading to the equation relating x and y coordinates. After manipulation, the locus can be derived as follows:
x² - 4y² - 16x²y² = 0
This represents the required locus of the midpoint of points A and B.
Conclusion
The correct option for the locus of the midpoint of AB is:
c) x² - 4y² - 16x²y² = 0
This indicates that the locus is a specific curve defined by the equation derived from the tangents to the hyperbola.
Identify the product for the following reaction.
CH ≡ CH + HOCI →- a)CH CI2 – C H O
- b)CH(OH) = CHCl
- c)CICH2CH2OH
- d)CH3COCI
Correct answer is option 'A'. Can you explain this answer?
Identify the product for the following reaction.
CH ≡ CH + HOCI →
CH ≡ CH + HOCI →
a)
CH CI2 – C H O
b)
CH(OH) = CHCl
c)
CICH2CH2OH
d)
CH3COCI

|
Learners Habitat answered |
HOCI → OH- + CI+
CI+ shall attack acetylene as an electrophile and OH- will end the addition with a nucleophilic addition. Therefore, the final reaction will be:
CH ≡ CH + 2 HOCI → CH (OH)2 - CH CI2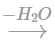 CH CI2 - C H O
CH CI2 - C H O
Unstable
CI+ shall attack acetylene as an electrophile and OH- will end the addition with a nucleophilic addition. Therefore, the final reaction will be:
CH ≡ CH + 2 HOCI → CH (OH)2 - CH CI2
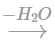 CH CI2 - C H O
CH CI2 - C H OUnstable
The compound that has the largest H-M-H bond angle (M = N, O, S, C) is- a)H2S
- b)CH4
- c)NH3
- d)H2O
Correct answer is option 'B'. Can you explain this answer?
The compound that has the largest H-M-H bond angle (M = N, O, S, C) is
a)
H2S
b)
CH4
c)
NH3
d)
H2O
|
|
Nidhi Choudhary answered |
Understanding H-M-H Bond Angles
The H-M-H bond angle varies depending on the central atom (M) and its hybridization. In this case, we are comparing compounds involving nitrogen (N), oxygen (O), sulfur (S), and carbon (C).
Factors Influencing Bond Angles
- Hybridization: The type of hybridization the atom undergoes plays a crucial role in determining the bond angle.
- Lone Pairs: The presence of lone pairs on the central atom can compress bond angles due to repulsion.
Analysis of Compounds
1. H₂S (Hydrogen Sulfide):
- Structure: Bent shape due to two lone pairs on sulfur.
- Bond Angle: Approximately 92°.
2. CH₄ (Methane):
- Structure: Tetrahedral shape with sp³ hybridization.
- Bond Angle: 109.5°.
3. NH₃ (Ammonia):
- Structure: Trigonal pyramidal due to one lone pair on nitrogen.
- Bond Angle: Approximately 107°.
4. H₂O (Water):
- Structure: Bent shape with two lone pairs on oxygen.
- Bond Angle: Approximately 104.5°.
Conclusion
- Among the given options, CH₄ (methane) has the largest H-M-H bond angle of 109.5° due to its tetrahedral geometry and absence of lone pairs.
- The other compounds, with their lone pairs and bent shapes, have smaller bond angles.
In summary, option 'B' (CH₄) indeed has the largest H-M-H bond angle, making it the correct answer to this question in the context of bond angles and molecular geometry.
The H-M-H bond angle varies depending on the central atom (M) and its hybridization. In this case, we are comparing compounds involving nitrogen (N), oxygen (O), sulfur (S), and carbon (C).
Factors Influencing Bond Angles
- Hybridization: The type of hybridization the atom undergoes plays a crucial role in determining the bond angle.
- Lone Pairs: The presence of lone pairs on the central atom can compress bond angles due to repulsion.
Analysis of Compounds
1. H₂S (Hydrogen Sulfide):
- Structure: Bent shape due to two lone pairs on sulfur.
- Bond Angle: Approximately 92°.
2. CH₄ (Methane):
- Structure: Tetrahedral shape with sp³ hybridization.
- Bond Angle: 109.5°.
3. NH₃ (Ammonia):
- Structure: Trigonal pyramidal due to one lone pair on nitrogen.
- Bond Angle: Approximately 107°.
4. H₂O (Water):
- Structure: Bent shape with two lone pairs on oxygen.
- Bond Angle: Approximately 104.5°.
Conclusion
- Among the given options, CH₄ (methane) has the largest H-M-H bond angle of 109.5° due to its tetrahedral geometry and absence of lone pairs.
- The other compounds, with their lone pairs and bent shapes, have smaller bond angles.
In summary, option 'B' (CH₄) indeed has the largest H-M-H bond angle, making it the correct answer to this question in the context of bond angles and molecular geometry.
During a negative beta decay,- a)An atomic electron is ejected
- b)An electron which is already present within the nucleus is ejected
- c)A neutron in the nucleus decays, emitting an electron Correct Answer.
- d)A part of the binding energy of the nucleus is converted into an electron
Correct answer is option 'C'. Can you explain this answer?
During a negative beta decay,
a)
An atomic electron is ejected
b)
An electron which is already present within the nucleus is ejected
c)
A neutron in the nucleus decays, emitting an electron Correct Answer.
d)
A part of the binding energy of the nucleus is converted into an electron

|
Manish Aggarwal answered |
Negative β decay is expressed by the following equation:
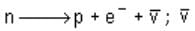 stands for antineutrino.
stands for antineutrino.
Hence, the correct choice is (c).
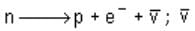 stands for antineutrino.
stands for antineutrino.Hence, the correct choice is (c).
The order of increasing acidic strengths of BF3, BCl3 and BBr3 is:- a)BBr3 < BCl3 < BF3
- b)BF3 < BCl3 < BBr3
- c)BBr3 < BF3 < BCl3
- d)BCl3 < BBr3 < BF3
Correct answer is option 'B'. Can you explain this answer?
The order of increasing acidic strengths of BF3, BCl3 and BBr3 is:
a)
BBr3 < BCl3 < BF3
b)
BF3 < BCl3 < BBr3
c)
BBr3 < BF3 < BCl3
d)
BCl3 < BBr3 < BF3

|
Tarun Kaushik answered |
The order of increasing acidic strengths of BF3, BCl3 and BBr3 is: BF3 < BCl3 < BBr3.
The extent of back bonding is maximum in BF3 and minimum in BBr3.
The extent of back bonding is maximum in BF3 and minimum in BBr3.
Which of the following gives benzoic acid on oxidation?- a)Chlorophenol
- b)Chlorotoluene
- c)Chlorobenzene
- d)Benzyl chloride
Correct answer is option 'D'. Can you explain this answer?
Which of the following gives benzoic acid on oxidation?
a)
Chlorophenol
b)
Chlorotoluene
c)
Chlorobenzene
d)
Benzyl chloride
|
|
Devika Das answered |
Explanation:
Benzyl chloride:
- Benzyl chloride (C₇H₇Cl) is the compound that gives benzoic acid on oxidation.
- Upon oxidation, benzyl chloride undergoes a series of reactions to form benzoic acid.
- The oxidation process involves breaking the C-Cl bond and forming a carboxylic acid group on the benzene ring.
Chlorophenol, Chlorotoluene, and Chlorobenzene:
- Chlorophenol, chlorotoluene, and chlorobenzene do not give benzoic acid on oxidation.
- These compounds have different chemical structures and do not undergo the same oxidation reactions as benzyl chloride to form benzoic acid.
Benzyl chloride:
- Benzyl chloride (C₇H₇Cl) is the compound that gives benzoic acid on oxidation.
- Upon oxidation, benzyl chloride undergoes a series of reactions to form benzoic acid.
- The oxidation process involves breaking the C-Cl bond and forming a carboxylic acid group on the benzene ring.
Chlorophenol, Chlorotoluene, and Chlorobenzene:
- Chlorophenol, chlorotoluene, and chlorobenzene do not give benzoic acid on oxidation.
- These compounds have different chemical structures and do not undergo the same oxidation reactions as benzyl chloride to form benzoic acid.
3 moles of P and 2 moles of Q are mixed, what will be their total vapour pressure in the solution if their partial vapour pressures are 80 and 60 torr respectively?
- a)80 torr
- b)140 torr
- c)72 torr
- d)70 torr
Correct answer is option 'C'. Can you explain this answer?
3 moles of P and 2 moles of Q are mixed, what will be their total vapour pressure in the solution if their partial vapour pressures are 80 and 60 torr respectively?
a)
80 torr
b)
140 torr
c)
72 torr
d)
70 torr

|
EduRev JEE answered |


Ptotal = pP + pQ = p∘PxP + p∘QxQ
= 80 x 0.6 + 60 x 0.4 = 72 torr.
Which of the following statement is correct regarding AC generators?- a)It converts mechanical energy into electrical energy.
- b)It works on the principle of Faraday's Law.
- c)Generated energy can be in the form of sinusoidal waveform.
- d)All of the above statements are correct regarding AC generators.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is correct regarding AC generators?
a)
It converts mechanical energy into electrical energy.
b)
It works on the principle of Faraday's Law.
c)
Generated energy can be in the form of sinusoidal waveform.
d)
All of the above statements are correct regarding AC generators.

|
Tarun Kaushik answered |
AC generator works on the principle of Faraday's Law. The AC Generator's input supply is mechanical energy supplied by steam turbines, gas turbines and combustion engines. The output is alternating electrical power in the form of alternating voltage and current.
AC generators convert mechanical energy into electrical energy. The generated energy is in the form of a sinusoidal waveform (alternating current).
So, all the statements regarding AC generators correct.
If 208 kJ/mol and 120 kJ/mol respectively are the observed heats of hydrogenation of cyclohexene and benzene, then the resonance energy of benzene in kJ/mol is (In integer)Correct answer is '152'. Can you explain this answer?
If 208 kJ/mol and 120 kJ/mol respectively are the observed heats of hydrogenation of cyclohexene and benzene, then the resonance energy of benzene in kJ/mol is (In integer)

|
EduRev JEE answered |
Resonance energy is the difference between the hydrogenation energy of three 'non-resonance' double bonds and the measured hydrogenation energy.
i.e. (3 × 120) − 208 = 152 kJ/mol
i.e. (3 × 120) − 208 = 152 kJ/mol
Pertaining to two planets, the ratio of escape velocities from respective surfaces is 1:2, the ratio of the time period of the same simple pendulum at their respective surfaces is 2:1 (in the same order). Then the find the ratio of their average densities.Correct answer is '4'. Can you explain this answer?
Pertaining to two planets, the ratio of escape velocities from respective surfaces is 1:2, the ratio of the time period of the same simple pendulum at their respective surfaces is 2:1 (in the same order). Then the find the ratio of their average densities.

|
Learners Habitat answered |
Time period of simple pendulum,
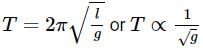
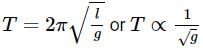
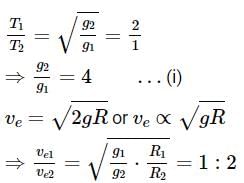
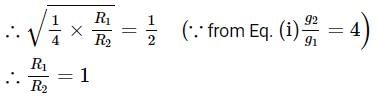
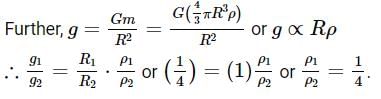
An organ pipe 40 cm long is open at both ends. The speed of sound in air is 360 ms-1.The frequency of the second harmonic is ___________ Hz.Correct answer is '900'. Can you explain this answer?
An organ pipe 40 cm long is open at both ends. The speed of sound in air is 360 ms-1.The frequency of the second harmonic is ___________ Hz.

|
EduRev JEE answered |
An organ pipe that is open at both ends resonates at all harmonics, including the fundamental (first harmonic), second harmonic, third harmonic, etc.
The frequency f of the n-th harmonic for a pipe open at both ends is given by:
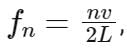
where:
n is the number of the harmonic,
v is the speed of sound, and
L is the length of the pipe.
n is the number of the harmonic,
v is the speed of sound, and
L is the length of the pipe.
To find the frequency of the second harmonic (n = 2) we can substitute the given values into the formula:
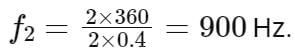
Therefore, the frequency of the second harmonic is 900 Hz.
In the second experiment of Faraday and Henry, the primary coil is connected to the galvanometer and the secondary coil is connected to a battery. If the primary coil is rotated about its axis, then:- a)The current will induce in the primary coil
- b)No current will induce in the primary coil
- c)Momentary current will induce in the primary coil
- d)Can't say
Correct answer is option 'B'. Can you explain this answer?
In the second experiment of Faraday and Henry, the primary coil is connected to the galvanometer and the secondary coil is connected to a battery. If the primary coil is rotated about its axis, then:
a)
The current will induce in the primary coil
b)
No current will induce in the primary coil
c)
Momentary current will induce in the primary coil
d)
Can't say

|
Tarun Kaushik answered |
According to the second experiment of Faraday and Henry, we can say that when a current-carrying coil is moved towards or away from another coil then an emf gets induced in the coil.
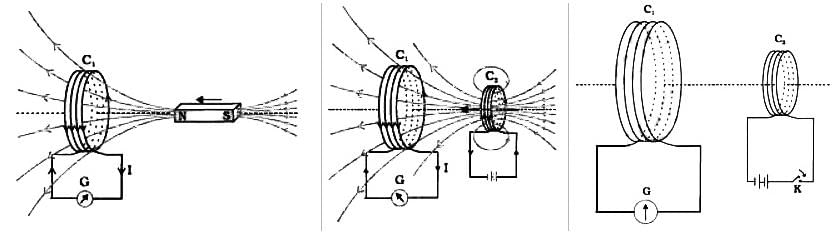
If the circuit is closed in which the coil is connected then a current will also get induced in the coil. The relative motion between the two coils is required to induce a current in the coil.

If the circuit is closed in which the coil is connected then a current will also get induced in the coil. The relative motion between the two coils is required to induce a current in the coil.
In the given case when the primary coil is rotated about its axis there will be no relative motion between the primary and the secondary coil.
Since, there is no relative motion between the primary and the secondary coil so no current will induce in the primary coil.
A box is floating in the river of speed 5m/s. The position of the block is shown in the figure at t = 0. A stone is thrown from point O at time t = 0 with a velocity 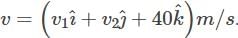 Find the value of v1, v2 such that the stone hits the box.
Find the value of v1, v2 such that the stone hits the box.
- a)v1 = 3 m/s v2 = 5 m/s
- b)v1 = 2 m/s v2 = 3 m/s
- c)v1 = 5 m/s v2 = 7.5 m/s
- d)v1 = 4 m/s v2 = 5 m/s
Correct answer is option 'C'. Can you explain this answer?
A box is floating in the river of speed 5m/s. The position of the block is shown in the figure at t = 0. A stone is thrown from point O at time t = 0 with a velocity 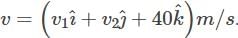 Find the value of v1, v2 such that the stone hits the box.
Find the value of v1, v2 such that the stone hits the box.
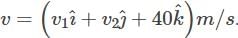 Find the value of v1, v2 such that the stone hits the box.
Find the value of v1, v2 such that the stone hits the box.
a)
v1 = 3 m/s v2 = 5 m/s
b)
v1 = 2 m/s v2 = 3 m/s
c)
v1 = 5 m/s v2 = 7.5 m/s
d)
v1 = 4 m/s v2 = 5 m/s

|
Infinity Academy answered |
Position vector of box and stone are
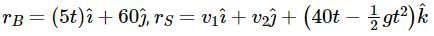
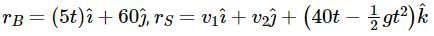
When stone hits the block,
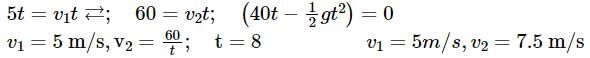
A 25 x 10-3 m3 volume cylinder is filled with 1 mol of O2 gas at room temperature (300 K). The molecular diameter of O2 and its root mean square speed, are found to be 0.3 nm and 200 m/s, respectively. What is the average collision rate (per second) for an O2 molecule?- a)~102
- b)~1010
- c)~1013
- d)~1011
Correct answer is option 'B'. Can you explain this answer?
A 25 x 10-3 m3 volume cylinder is filled with 1 mol of O2 gas at room temperature (300 K). The molecular diameter of O2 and its root mean square speed, are found to be 0.3 nm and 200 m/s, respectively. What is the average collision rate (per second) for an O2 molecule?
a)
~102
b)
~10
10
c)
~1013
d)
~1011
|
|
Harshad Das answered |
To determine the average collision rate per second for an O2 molecule in the given scenario, we need to consider the volume of the cylinder, the molecular diameter of O2, and its root mean square speed.
1. Calculate the number of O2 molecules:
Given that 1 mole of O2 gas is present, we can use Avogadro's number (6.022 × 10^23 mol^-1) to calculate the number of O2 molecules. Therefore, the number of O2 molecules is:
Number of O2 molecules = 1 mol × 6.022 × 10^23 mol^-1 = 6.022 × 10^23 molecules
2. Determine the volume occupied by a single O2 molecule:
The volume occupied by a single O2 molecule can be approximated as a sphere with a diameter equal to the molecular diameter of O2 (0.3 nm). Using the formula for the volume of a sphere, we can calculate the volume occupied by a single O2 molecule as:
Volume of a single O2 molecule = (4/3)π(r^3) = (4/3)π(0.3/2)^3 = 0.014137 nm^3
3. Calculate the total volume occupied by all O2 molecules:
The total volume occupied by all O2 molecules can be calculated by multiplying the volume of a single molecule by the number of molecules:
Total volume occupied by O2 molecules = Volume of a single O2 molecule × Number of O2 molecules
Total volume occupied by O2 molecules = 0.014137 nm^3 × 6.022 × 10^23 molecules = 8.507 × 10^21 nm^3
4. Convert the total volume to cubic meters:
To calculate the average collision rate per second, we need to convert the total volume occupied by O2 molecules to cubic meters:
Total volume occupied by O2 molecules = 8.507 × 10^21 nm^3
Converting nm^3 to m^3: 1 nm^3 = (10^-9 m)^3 = 10^-27 m^3
Total volume occupied by O2 molecules = 8.507 × 10^21 nm^3 × 10^-27 m^3/nm^3 = 8.507 × 10^-6 m^3
5. Determine the average collision rate per second:
The average collision rate per second can be calculated using the formula:
Collision rate = (Total volume occupied by O2 molecules) / (Volume of the cylinder × Root mean square speed of O2)
Collision rate = 8.507 × 10^-6 m^3 / (25 × 10^-3 m^3 × 200 m/s)
Collision rate ≈ 1.7014 × 10^-2 s^-1
Since the collision rate is approximately 1.7014 × 10^-2 s^-1, which is of the order of 10^0, the correct answer is approximately 10^0, or option (b) ~10^10.
1. Calculate the number of O2 molecules:
Given that 1 mole of O2 gas is present, we can use Avogadro's number (6.022 × 10^23 mol^-1) to calculate the number of O2 molecules. Therefore, the number of O2 molecules is:
Number of O2 molecules = 1 mol × 6.022 × 10^23 mol^-1 = 6.022 × 10^23 molecules
2. Determine the volume occupied by a single O2 molecule:
The volume occupied by a single O2 molecule can be approximated as a sphere with a diameter equal to the molecular diameter of O2 (0.3 nm). Using the formula for the volume of a sphere, we can calculate the volume occupied by a single O2 molecule as:
Volume of a single O2 molecule = (4/3)π(r^3) = (4/3)π(0.3/2)^3 = 0.014137 nm^3
3. Calculate the total volume occupied by all O2 molecules:
The total volume occupied by all O2 molecules can be calculated by multiplying the volume of a single molecule by the number of molecules:
Total volume occupied by O2 molecules = Volume of a single O2 molecule × Number of O2 molecules
Total volume occupied by O2 molecules = 0.014137 nm^3 × 6.022 × 10^23 molecules = 8.507 × 10^21 nm^3
4. Convert the total volume to cubic meters:
To calculate the average collision rate per second, we need to convert the total volume occupied by O2 molecules to cubic meters:
Total volume occupied by O2 molecules = 8.507 × 10^21 nm^3
Converting nm^3 to m^3: 1 nm^3 = (10^-9 m)^3 = 10^-27 m^3
Total volume occupied by O2 molecules = 8.507 × 10^21 nm^3 × 10^-27 m^3/nm^3 = 8.507 × 10^-6 m^3
5. Determine the average collision rate per second:
The average collision rate per second can be calculated using the formula:
Collision rate = (Total volume occupied by O2 molecules) / (Volume of the cylinder × Root mean square speed of O2)
Collision rate = 8.507 × 10^-6 m^3 / (25 × 10^-3 m^3 × 200 m/s)
Collision rate ≈ 1.7014 × 10^-2 s^-1
Since the collision rate is approximately 1.7014 × 10^-2 s^-1, which is of the order of 10^0, the correct answer is approximately 10^0, or option (b) ~10^10.
Out of 11 consecutive natural numbers, if three numbers are selected at random (without repetition), then the probability that they are in A.P. with positive common difference, is:- a)5/101
- b)10/99
- c)5/33
- d)15/101
Correct answer is option 'C'. Can you explain this answer?
Out of 11 consecutive natural numbers, if three numbers are selected at random (without repetition), then the probability that they are in A.P. with positive common difference, is:
a)
5/101
b)
10/99
c)
5/33
d)
15/101
|
|
Manasa Bajaj answered |
Probability of selecting three numbers in A.P. with positive common difference can be calculated by dividing the favorable outcomes by the total number of outcomes.
Let's consider the given set of 11 consecutive natural numbers: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11.
To form an arithmetic progression (A.P.) with positive common difference, we need to select three numbers such that the middle number is greater than the first number and the third number is greater than the middle number.
Let's count the favorable outcomes:
- We have a total of 11 natural numbers to choose from as the first number.
- For the second number, we can choose any number from the remaining 10 numbers.
- For the third number, we can choose any number from the remaining 9 numbers.
So, the total number of favorable outcomes is 11 * 10 * 9 = 990.
To calculate the total number of outcomes, we need to calculate the number of ways we can choose 3 numbers from a set of 11 numbers, without considering the order. This can be calculated using the combination formula:
Total number of outcomes = C(11, 3) = (11!)/(3!(11-3)!) = 165.
Therefore, the probability of selecting three numbers in A.P. with positive common difference is:
Probability = (Number of favorable outcomes)/(Total number of outcomes) = 990/165 = 6.
Simplifying further, we get:
Probability = 6/165 = 2/55.
However, the given options do not include 2/55 as a choice. So, there must be a mistake in the options provided.
Hence, the correct answer cannot be determined from the given options.
Let's consider the given set of 11 consecutive natural numbers: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11.
To form an arithmetic progression (A.P.) with positive common difference, we need to select three numbers such that the middle number is greater than the first number and the third number is greater than the middle number.
Let's count the favorable outcomes:
- We have a total of 11 natural numbers to choose from as the first number.
- For the second number, we can choose any number from the remaining 10 numbers.
- For the third number, we can choose any number from the remaining 9 numbers.
So, the total number of favorable outcomes is 11 * 10 * 9 = 990.
To calculate the total number of outcomes, we need to calculate the number of ways we can choose 3 numbers from a set of 11 numbers, without considering the order. This can be calculated using the combination formula:
Total number of outcomes = C(11, 3) = (11!)/(3!(11-3)!) = 165.
Therefore, the probability of selecting three numbers in A.P. with positive common difference is:
Probability = (Number of favorable outcomes)/(Total number of outcomes) = 990/165 = 6.
Simplifying further, we get:
Probability = 6/165 = 2/55.
However, the given options do not include 2/55 as a choice. So, there must be a mistake in the options provided.
Hence, the correct answer cannot be determined from the given options.
The integrating factor of dy/dx - 3y/x = x3 sin x is- a)1/x2
- b)1/x3
- c)x
- d)x2
Correct answer is option 'B'. Can you explain this answer?
The integrating factor of dy/dx - 3y/x = x3 sin x is
a)
1/x2
b)
1/x3
c)
x
d)
x2

|
Tarun Kaushik answered |
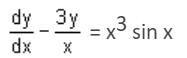
This is a linear differential equation of the form dy/dx + Px = Q.
P = -3/x
Integrating Factor (I.F.) =
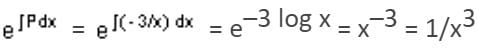
Find the coefficient of x4 in the expansion of (1 + x + x2)10 .Correct answer is '615'. Can you explain this answer?
Find the coefficient of x4 in the expansion of (1 + x + x2)10 .

|
Tarun Kaushik answered |
General term of the given expression is,
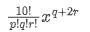
Here, q + 2r = 4
For p = 6, q = 4, r = 0, coefficient = 10!/[6! x 4!] = 210
For p = 7, q = 2, r = 1, coefficient = 10! /[7! x 2! x 1!] = 360
For p = 8, q = 0, r = 2, coefficient = 10!/[ 8! x 2!] = 45
Therefore, sum = 615
The equation of a plane containing the line of intersection of the planes 2x - y - 4 = 0 and y + 2z - 4 = 0 and passing through the point (1, 1, 0) is:- a)x + 3y + z = 4
- b)x - y - z = 0
- c)x - 3y - 2z = -2
- d)2x - z = 2
Correct answer is option 'B'. Can you explain this answer?
The equation of a plane containing the line of intersection of the planes 2x - y - 4 = 0 and y + 2z - 4 = 0 and passing through the point (1, 1, 0) is:
a)
x + 3y + z = 4
b)
x - y - z = 0
c)
x - 3y - 2z = -2
d)
2x - z = 2

|
EduRev JEE answered |
The required plane is:
(2x - y - 4) + λ(y + 2z - 4) = 0 ... (1)
It passes through (1, 1, 0).
⇒(2−1−4) +λ(1 − 4) = 0
⇒ −3 − 3λ = 0 ⇒ λ = −1
Substitute the value of λ=−1 in equation (1).
The required equation of plane is:
x−y−z=0
(2x - y - 4) + λ(y + 2z - 4) = 0 ... (1)
It passes through (1, 1, 0).
⇒(2−1−4) +λ(1 − 4) = 0
⇒ −3 − 3λ = 0 ⇒ λ = −1
Substitute the value of λ=−1 in equation (1).
The required equation of plane is:
x−y−z=0
When a conductor is placed in an electric field; its free charge carriers adjust itself in order to oppose the electric field. This happen until- a)Both the fields cancel out each other
- b)Induced field become more than external field
- c)External field reach the maximum value
- d)Induced field reach the maximum value
Correct answer is option 'A'. Can you explain this answer?
When a conductor is placed in an electric field; its free charge carriers adjust itself in order to oppose the electric field. This happen until
a)
Both the fields cancel out each other
b)
Induced field become more than external field
c)
External field reach the maximum value
d)
Induced field reach the maximum value
|
|
Tanishq Ghoshal answered |
Introduction:
When a conductor is placed in an electric field, its free charge carriers (electrons or ions) adjust themselves in order to oppose the electric field. This behavior is known as the shielding effect or the screening effect. The charge carriers redistribute themselves in such a way that the net electric field inside the conductor becomes zero.
Explanation:
When a conductor is placed in an electric field, the following process takes place:
1. Redistribution of charge carriers:
The free charge carriers in the conductor (electrons in metals or ions in electrolytes) start to redistribute themselves in response to the electric field. The negative charge carriers (electrons or anions) move towards the side of the conductor facing the electric field, while the positive charge carriers (holes or cations) move towards the opposite side.
2. Induced electric field:
As the charge carriers redistribute, an induced electric field is created within the conductor. This induced electric field is opposite in direction to the external electric field. The induced electric field exerts a force on the free charge carriers, opposing the motion caused by the external electric field.
3. Equilibrium state:
The redistribution of charge carriers continues until the induced electric field cancels out the external electric field completely. At this point, the net electric field inside the conductor becomes zero. The conductor has reached an equilibrium state where the electric field inside is cancelled out by the induced electric field.
Conclusion:
The correct answer is option 'A' - Both the fields cancel out each other. When a conductor is placed in an electric field, its free charge carriers adjust themselves to oppose the electric field. This adjustment continues until the induced electric field cancels out the external electric field, resulting in a net electric field of zero inside the conductor.
When a conductor is placed in an electric field, its free charge carriers (electrons or ions) adjust themselves in order to oppose the electric field. This behavior is known as the shielding effect or the screening effect. The charge carriers redistribute themselves in such a way that the net electric field inside the conductor becomes zero.
Explanation:
When a conductor is placed in an electric field, the following process takes place:
1. Redistribution of charge carriers:
The free charge carriers in the conductor (electrons in metals or ions in electrolytes) start to redistribute themselves in response to the electric field. The negative charge carriers (electrons or anions) move towards the side of the conductor facing the electric field, while the positive charge carriers (holes or cations) move towards the opposite side.
2. Induced electric field:
As the charge carriers redistribute, an induced electric field is created within the conductor. This induced electric field is opposite in direction to the external electric field. The induced electric field exerts a force on the free charge carriers, opposing the motion caused by the external electric field.
3. Equilibrium state:
The redistribution of charge carriers continues until the induced electric field cancels out the external electric field completely. At this point, the net electric field inside the conductor becomes zero. The conductor has reached an equilibrium state where the electric field inside is cancelled out by the induced electric field.
Conclusion:
The correct answer is option 'A' - Both the fields cancel out each other. When a conductor is placed in an electric field, its free charge carriers adjust themselves to oppose the electric field. This adjustment continues until the induced electric field cancels out the external electric field, resulting in a net electric field of zero inside the conductor.
Let AD and BC be two vertical poles at A and B, respectively, on a horizontal ground. If AD = 8 m, BC = 11 m and AB = 10 m, then the distance (in metres) of a point M on AB from the point A such that MD2 + MC2 is minimum is ________. (in integer)Correct answer is '5'. Can you explain this answer?
Let AD and BC be two vertical poles at A and B, respectively, on a horizontal ground. If AD = 8 m, BC = 11 m and AB = 10 m, then the distance (in metres) of a point M on AB from the point A such that MD2 + MC2 is minimum is ________. (in integer)

|
Imk Pathsala answered |
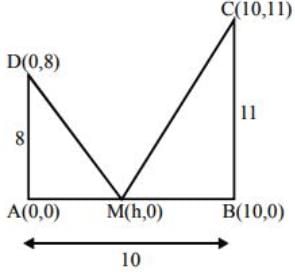
(MD)2 + (MC)2 = h2 + 64 + (h - 10)2 + 121
= 2h2 - 20h + 64 + 100 + 121
= 2(h2 - 10h) + 285
= 2(h - 5)2 + 235
It is minimum if h = 5.
= 2h2 - 20h + 64 + 100 + 121
= 2(h2 - 10h) + 285
= 2(h - 5)2 + 235
It is minimum if h = 5.
An aqueous solution contains 0.10 M H2S and 0.20 M HCI. If the equilibrium constant for the formation of HS- from H2S is 1 .0 x 10-7 and that of S2- from HS- ions is 1.2 x 10-13, then the concentration of S2- ions in aqueous solution is- a)5 x 10-8
- b)3 x 10-20
- c)6 x 10-21
- d)5 x 10-19
Correct answer is option 'B'. Can you explain this answer?
An aqueous solution contains 0.10 M H2S and 0.20 M HCI. If the equilibrium constant for the formation of HS- from H2S is 1 .0 x 10-7 and that of S2- from HS- ions is 1.2 x 10-13, then the concentration of S2- ions in aqueous solution is
a)
5 x 10-8
b)
3 x 10-20
c)
6 x 10-21
d)
5 x 10-19
|
|
Meghana Sen answered |
To find the concentration of S2- ions in the aqueous solution, we need to consider the equilibrium reactions involving H2S, HS-, and S2- ions.
Given:
[H2S] = 0.10 M
[HCl] = 0.20 M
Equilibrium constant for the formation of HS- from H2S (K1) = 1.0 x 10^-7
Equilibrium constant for the formation of S2- from HS- (K2) = 1.2 x 10^-13
First, let's write the equilibrium reactions:
1) H2S ⇌ HS- + H+
2) HS- ⇌ S2- + H+
Let's assume the concentration of HS- ions formed be x M. Then, the concentration of H+ ions formed will also be x M.
Using the equilibrium constant expression for the reaction 1, we can write:
K1 = [HS-][H+]/[H2S]
Substituting the values given:
1.0 x 10^-7 = x * x / (0.10 - x)
Since the concentration of H2S is much greater than x, we can assume that 0.10 - x ≈ 0.10. Simplifying the equation:
1.0 x 10^-7 = x^2 / 0.10
x^2 = 1.0 x 10^-8
x = √(1.0 x 10^-8)
x ≈ 1.0 x 10^-4 M
Now, using the equilibrium constant expression for the reaction 2, we can write:
K2 = [S2-][H+]/[HS-]
Substituting the values given:
1.2 x 10^-13 = [S2-] * x / (1.0 x 10^-4)
[S2-] = (1.2 x 10^-13) * (1.0 x 10^-4) / x
[S2-] ≈ 1.2 x 10^-17 / (1.0 x 10^-4)
[S2-] ≈ 1.2 x 10^-21 M
Therefore, the concentration of S2- ions in the aqueous solution is approximately 1.2 x 10^-21 M, which corresponds to option B.
Given:
[H2S] = 0.10 M
[HCl] = 0.20 M
Equilibrium constant for the formation of HS- from H2S (K1) = 1.0 x 10^-7
Equilibrium constant for the formation of S2- from HS- (K2) = 1.2 x 10^-13
First, let's write the equilibrium reactions:
1) H2S ⇌ HS- + H+
2) HS- ⇌ S2- + H+
Let's assume the concentration of HS- ions formed be x M. Then, the concentration of H+ ions formed will also be x M.
Using the equilibrium constant expression for the reaction 1, we can write:
K1 = [HS-][H+]/[H2S]
Substituting the values given:
1.0 x 10^-7 = x * x / (0.10 - x)
Since the concentration of H2S is much greater than x, we can assume that 0.10 - x ≈ 0.10. Simplifying the equation:
1.0 x 10^-7 = x^2 / 0.10
x^2 = 1.0 x 10^-8
x = √(1.0 x 10^-8)
x ≈ 1.0 x 10^-4 M
Now, using the equilibrium constant expression for the reaction 2, we can write:
K2 = [S2-][H+]/[HS-]
Substituting the values given:
1.2 x 10^-13 = [S2-] * x / (1.0 x 10^-4)
[S2-] = (1.2 x 10^-13) * (1.0 x 10^-4) / x
[S2-] ≈ 1.2 x 10^-17 / (1.0 x 10^-4)
[S2-] ≈ 1.2 x 10^-21 M
Therefore, the concentration of S2- ions in the aqueous solution is approximately 1.2 x 10^-21 M, which corresponds to option B.
In KO2, the nature of oxygen species and the oxidation state of oxygen atom are (respectively)- a)Superoxide and -1
- b)Oxide and -2
- c)Peroxide and -1/2
- d)Superoxide and -1/2
Correct answer is option 'D'. Can you explain this answer?
In KO2, the nature of oxygen species and the oxidation state of oxygen atom are (respectively)
a)
Superoxide and -1
b)
Oxide and -2
c)
Peroxide and -1/2
d)
Superoxide and -1/2

|
Ambition Institute answered |
In KO2, the nature of oxygen species and the oxidation state of oxygen atom are, superoxide and −1/2 respectively.
Superoxide ion is O-2.
Let X be an oxidation state of oxygen. The oxidation state of K is +1.
+ 1 + 2(X) = 0
2X = - 1
X = -1/2
Superoxide ion is O-2.
Let X be an oxidation state of oxygen. The oxidation state of K is +1.
+ 1 + 2(X) = 0
2X = - 1
X = -1/2
If |z - 3 + 2i| ≤ 4, then the difference between the greatest value and the least value of |z| is:- a)4 + √13
- b)2√13
- c)√13
- d)8
Correct answer is option 'A'. Can you explain this answer?
If |z - 3 + 2i| ≤ 4, then the difference between the greatest value and the least value of |z| is:
a)
4 + √13
b)
2√13
c)
√13
d)
8
|
|
Anshika Patel answered |
- Given information:
Given |z - 3 + 2i| ≤ 4.
- Definition of Absolute Value:
The absolute value of a complex number z = a + bi is |z| = sqrt(a^2 + b^2).
- Expressing the Given Inequality:
We can rewrite the given inequality as |z - (3 - 2i)| ≤ 4.
This means that the distance between z and (3 - 2i) is less than or equal to 4.
- Geometric Interpretation:
This inequality represents a circle centered at (3, -2) with a radius of 4 in the complex plane.
- Maximum and Minimum Values:
The maximum value of |z| occurs on the circle when z is farthest away from the center, which is at the point of intersection of the circle with the line passing through the origin and the center of the circle.
The minimum value of |z| occurs when z is closest to the center, which is at the point (3 - 2i).
- Calculating the Values:
The maximum value of |z| can be found by calculating the distance between the point of intersection and the origin. This distance is 4 + sqrt(13).
The minimum value of |z| is the distance between (3 - 2i) and the origin, which is sqrt(13).
- Calculating the Difference:
The difference between the maximum and minimum values of |z| is:
(4 + sqrt(13)) - sqrt(13) = 4 + sqrt(13).
Therefore, the correct answer is option 'A' (4 + sqrt(13)).
Given |z - 3 + 2i| ≤ 4.
- Definition of Absolute Value:
The absolute value of a complex number z = a + bi is |z| = sqrt(a^2 + b^2).
- Expressing the Given Inequality:
We can rewrite the given inequality as |z - (3 - 2i)| ≤ 4.
This means that the distance between z and (3 - 2i) is less than or equal to 4.
- Geometric Interpretation:
This inequality represents a circle centered at (3, -2) with a radius of 4 in the complex plane.
- Maximum and Minimum Values:
The maximum value of |z| occurs on the circle when z is farthest away from the center, which is at the point of intersection of the circle with the line passing through the origin and the center of the circle.
The minimum value of |z| occurs when z is closest to the center, which is at the point (3 - 2i).
- Calculating the Values:
The maximum value of |z| can be found by calculating the distance between the point of intersection and the origin. This distance is 4 + sqrt(13).
The minimum value of |z| is the distance between (3 - 2i) and the origin, which is sqrt(13).
- Calculating the Difference:
The difference between the maximum and minimum values of |z| is:
(4 + sqrt(13)) - sqrt(13) = 4 + sqrt(13).
Therefore, the correct answer is option 'A' (4 + sqrt(13)).
The most suitable reagent for the conversion of R - CH2 - OH → R - CHO is- a)CrO3
- b)PCC (Pyridinium Chlorochromate)
- c)KMnO4
- d)K2Cr2O7
Correct answer is option 'B'. Can you explain this answer?
The most suitable reagent for the conversion of R - CH2 - OH → R - CHO is
a)
CrO3
b)
PCC (Pyridinium Chlorochromate)
c)
KMnO4
d)
K2Cr2O7

|
Ambition Institute answered |
Pyridinium chlorochromate (PCC) is a mild oxidising agent. It can easily convert primary alcohols into aldehydes.

While others are strong oxidising agents that convert alcohols into carboxylic acid.

While others are strong oxidising agents that convert alcohols into carboxylic acid.
For producing the effective collisions, the colliding molecules must posses:- a)First order reactions
- b)Second order reactions
- c)Bimolecular reactions
- d)Zeroth order reactions
Correct answer is option 'C'. Can you explain this answer?
For producing the effective collisions, the colliding molecules must posses:
a)
First order reactions
b)
Second order reactions
c)
Bimolecular reactions
d)
Zeroth order reactions
|
|
Rajeev Choudhary answered |
To understand why the correct answer is option C (bimolecular reactions), let's first define what effective collisions are in the context of chemical reactions.
Effective collisions are those collisions between molecules that result in a chemical reaction taking place. Not all collisions between molecules lead to a reaction, as certain conditions must be met for a reaction to occur. One of these conditions is that the colliding molecules must possess enough energy to overcome the activation energy barrier for the reaction.
Now let's look at the different options provided and analyze why option C is the correct answer.
a) First order reactions:
First order reactions refer to reactions where the rate of the reaction depends on the concentration of a single reactant. While the concentration of reactants can influence the rate of a reaction, it does not necessarily determine whether an effective collision occurs or not. Therefore, first order reactions alone do not guarantee effective collisions.
b) Second order reactions:
Second order reactions refer to reactions where the rate of the reaction depends on the concentration of two reactants. Similar to first order reactions, the concentration of reactants can influence the rate of a reaction, but it does not determine whether an effective collision occurs or not. Therefore, second order reactions alone do not guarantee effective collisions.
c) Bimolecular reactions:
Bimolecular reactions refer to reactions involving the collision between two molecules. In order for effective collisions to occur, the colliding molecules must possess enough energy to overcome the activation energy barrier. Bimolecular reactions have a higher likelihood of leading to effective collisions because the collision between two molecules increases the chances of having enough energy to overcome the activation energy barrier.
d) Zeroth order reactions:
Zeroth order reactions refer to reactions where the rate of the reaction is independent of the concentration of the reactants. In these reactions, the concentration of the reactants does not influence the rate of the reaction, and therefore, it does not determine whether effective collisions occur or not.
In conclusion, the correct answer is option C (bimolecular reactions) because the collision between two molecules increases the chances of having enough energy to overcome the activation energy barrier, leading to effective collisions.
Effective collisions are those collisions between molecules that result in a chemical reaction taking place. Not all collisions between molecules lead to a reaction, as certain conditions must be met for a reaction to occur. One of these conditions is that the colliding molecules must possess enough energy to overcome the activation energy barrier for the reaction.
Now let's look at the different options provided and analyze why option C is the correct answer.
a) First order reactions:
First order reactions refer to reactions where the rate of the reaction depends on the concentration of a single reactant. While the concentration of reactants can influence the rate of a reaction, it does not necessarily determine whether an effective collision occurs or not. Therefore, first order reactions alone do not guarantee effective collisions.
b) Second order reactions:
Second order reactions refer to reactions where the rate of the reaction depends on the concentration of two reactants. Similar to first order reactions, the concentration of reactants can influence the rate of a reaction, but it does not determine whether an effective collision occurs or not. Therefore, second order reactions alone do not guarantee effective collisions.
c) Bimolecular reactions:
Bimolecular reactions refer to reactions involving the collision between two molecules. In order for effective collisions to occur, the colliding molecules must possess enough energy to overcome the activation energy barrier. Bimolecular reactions have a higher likelihood of leading to effective collisions because the collision between two molecules increases the chances of having enough energy to overcome the activation energy barrier.
d) Zeroth order reactions:
Zeroth order reactions refer to reactions where the rate of the reaction is independent of the concentration of the reactants. In these reactions, the concentration of the reactants does not influence the rate of the reaction, and therefore, it does not determine whether effective collisions occur or not.
In conclusion, the correct answer is option C (bimolecular reactions) because the collision between two molecules increases the chances of having enough energy to overcome the activation energy barrier, leading to effective collisions.
Consider separate solutions of 0.500 M C2H5OH (aq), 0.100 M Mg3(PO4)2 (aq), 0.250 M KBr (aq) and 0.125 M Na3PO4 (aq) at 25oC. Which statement is true about these solutions, assuming all salts to be strong electrolytes?- a)0.125 M Na3PO4 (aq) has the highest osmotic pressure.
- b)0.500 M C2H5OH (aq) has the highest osmotic pressure.
- c)They all have the same osmotic pressure.
- d)0.100 M Mg3(PO4)2 (aq) has the highest osmotic pressure.
Correct answer is option 'C'. Can you explain this answer?
Consider separate solutions of 0.500 M C2H5OH (aq), 0.100 M Mg3(PO4)2 (aq), 0.250 M KBr (aq) and 0.125 M Na3PO4 (aq) at 25oC. Which statement is true about these solutions, assuming all salts to be strong electrolytes?
a)
0.125 M Na3PO4 (aq) has the highest osmotic pressure.
b)
0.500 M C2H5OH (aq) has the highest osmotic pressure.
c)
They all have the same osmotic pressure.
d)
0.100 M Mg3(PO4)2 (aq) has the highest osmotic pressure.
|
|
Anuj Choudhary answered |
Explanation:
Understanding Osmotic Pressure:
Osmotic pressure is a colligative property that depends on the concentration of solute particles in a solution. It is directly proportional to the molarity of the solution.
Comparing Osmotic Pressures:
- 0.500 M C2H5OH (aq): This solution contains only one type of solute particles (ethanol molecules), so its osmotic pressure will be proportional to its molarity.
- 0.100 M Mg3(PO4)2(aq): This solution contains magnesium and phosphate ions, resulting in a total of 5 solute particles (3 Mg2+ and 2 PO4^3-) for each formula unit dissolved. Therefore, it will have a higher osmotic pressure compared to a solution with only one type of solute particles.
- 0.250 M KBr (aq): This solution contains potassium and bromide ions, resulting in a total of 2 solute particles for each formula unit dissolved.
- 0.125 M Na3PO4(aq): This solution contains sodium and phosphate ions, resulting in a total of 4 solute particles for each formula unit dissolved.
Conclusion:
Since osmotic pressure is directly proportional to the molarity of the solution and all solutions have different molarities and types of solute particles, the osmotic pressures of these solutions will be different. Therefore, option 'c' is incorrect, and the correct answer is that they all have different osmotic pressures.
Understanding Osmotic Pressure:
Osmotic pressure is a colligative property that depends on the concentration of solute particles in a solution. It is directly proportional to the molarity of the solution.
Comparing Osmotic Pressures:
- 0.500 M C2H5OH (aq): This solution contains only one type of solute particles (ethanol molecules), so its osmotic pressure will be proportional to its molarity.
- 0.100 M Mg3(PO4)2(aq): This solution contains magnesium and phosphate ions, resulting in a total of 5 solute particles (3 Mg2+ and 2 PO4^3-) for each formula unit dissolved. Therefore, it will have a higher osmotic pressure compared to a solution with only one type of solute particles.
- 0.250 M KBr (aq): This solution contains potassium and bromide ions, resulting in a total of 2 solute particles for each formula unit dissolved.
- 0.125 M Na3PO4(aq): This solution contains sodium and phosphate ions, resulting in a total of 4 solute particles for each formula unit dissolved.
Conclusion:
Since osmotic pressure is directly proportional to the molarity of the solution and all solutions have different molarities and types of solute particles, the osmotic pressures of these solutions will be different. Therefore, option 'c' is incorrect, and the correct answer is that they all have different osmotic pressures.
What speed should a galaxy move with respect to us so that the sodium line at 589.0 nm is observed at 589.6 nm?- a)1.06 x 105 m/s
- b)2.06 x 105 m/s
- c)3.06 x 105 m/s
- d)4.06 x 105 m/s
Correct answer is option 'C'. Can you explain this answer?
What speed should a galaxy move with respect to us so that the sodium line at 589.0 nm is observed at 589.6 nm?
a)
1.06 x 105 m/s
b)
2.06 x 105 m/s
c)
3.06 x 105 m/s
d)
4.06 x 105 m/s
|
|
Ipsita Banerjee answered |
Understanding the Doppler Effect
The observed change in wavelength due to the relative motion between the source of light (in this case, a galaxy) and the observer (us) is explained by the Doppler Effect. When a galaxy is moving away from us, the light it emits is redshifted, which means the wavelength increases.
Calculation of Redshift
1. Initial and Observed Wavelengths:
- The sodium line has an initial wavelength (λ₀) of 589.0 nm.
- The observed wavelength (λ) is 589.6 nm.
2. Calculating the Change in Wavelength:
- Δλ = λ - λ₀ = 589.6 nm - 589.0 nm = 0.6 nm.
3. Redshift (z) Formula:
- The redshift can be expressed as:
z = Δλ / λ₀.
- Plugging in the values:
z = 0.6 nm / 589.0 nm ≈ 0.001017.
Relating Redshift to Velocity
Using the formula for redshift in terms of velocity (v) for non-relativistic speeds:
z ≈ v / c, where c is the speed of light (approximately 3 x 10^8 m/s).
4. Calculating Velocity:
- Rearranging gives us:
v = z * c.
- Substituting the values:
v ≈ 0.001017 * 3 x 10^8 m/s ≈ 3.06 x 10^5 m/s.
Conclusion
The galaxy must be moving away from us at a speed of approximately 3.06 x 10^5 m/s for the sodium line at 589.0 nm to be observed at 589.6 nm, confirming option 'C' as the correct answer.
The observed change in wavelength due to the relative motion between the source of light (in this case, a galaxy) and the observer (us) is explained by the Doppler Effect. When a galaxy is moving away from us, the light it emits is redshifted, which means the wavelength increases.
Calculation of Redshift
1. Initial and Observed Wavelengths:
- The sodium line has an initial wavelength (λ₀) of 589.0 nm.
- The observed wavelength (λ) is 589.6 nm.
2. Calculating the Change in Wavelength:
- Δλ = λ - λ₀ = 589.6 nm - 589.0 nm = 0.6 nm.
3. Redshift (z) Formula:
- The redshift can be expressed as:
z = Δλ / λ₀.
- Plugging in the values:
z = 0.6 nm / 589.0 nm ≈ 0.001017.
Relating Redshift to Velocity
Using the formula for redshift in terms of velocity (v) for non-relativistic speeds:
z ≈ v / c, where c is the speed of light (approximately 3 x 10^8 m/s).
4. Calculating Velocity:
- Rearranging gives us:
v = z * c.
- Substituting the values:
v ≈ 0.001017 * 3 x 10^8 m/s ≈ 3.06 x 10^5 m/s.
Conclusion
The galaxy must be moving away from us at a speed of approximately 3.06 x 10^5 m/s for the sodium line at 589.0 nm to be observed at 589.6 nm, confirming option 'C' as the correct answer.
When 2-butyne is treated with H2/Lindlar's catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?- a)X will have higher dipole moment and higher boiling point than Y.
- b)Y will have higher dipole moment and lower boiling point than X.
- c)Y will have higher dipole moment and higher boiling point than X.
- d)X will have lower dipole moment and lower boiling point than Y.
Correct answer is option 'A'. Can you explain this answer?
When 2-butyne is treated with H2/Lindlar's catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?
a)
X will have higher dipole moment and higher boiling point than Y.
b)
Y will have higher dipole moment and lower boiling point than X.
c)
Y will have higher dipole moment and higher boiling point than X.
d)
X will have lower dipole moment and lower boiling point than Y.
|
|
Lekshmi Joshi answered |
Answer:
Introduction:
The given question involves the reaction of 2-butyne with two different reagents, H2/Lindlars catalyst and Na/liq. NH3. We need to determine the major products formed in each case and compare their dipole moments and boiling points.
Reaction with H2/Lindlars catalyst:
When 2-butyne is treated with H2/Lindlars catalyst, it undergoes hydrogenation to produce compound X as the major product. The Lindlars catalyst is a poisoned catalyst that selectively reduces alkynes to cis-alkenes without further reduction to alkanes.
Reaction with Na/liq. NH3:
When 2-butyne is treated with Na/liq. NH3, it undergoes a reaction known as dissolving metal reduction. The sodium metal dissolves in liquid ammonia to form the deep blue color sodium-ammonia solution, which acts as a strong reducing agent. In this reaction, the triple bond of 2-butyne is reduced to form compound Y as the major product.
Comparison between compound X and Y:
To determine the dipole moment and boiling point of compounds X and Y, we need to analyze their structures.
Compound X:
Compound X is the cis-alkene formed by the hydrogenation of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
H
The dipole moment of compound X is expected to be low since it is a symmetric molecule with no significant charge separation. The boiling point of compound X will be determined by intermolecular forces such as London dispersion forces, which are generally weaker for alkenes compared to alcohols or carboxylic acids.
Compound Y:
Compound Y is the product of dissolving metal reduction of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
Na
The presence of the metal atom (Na) in compound Y can lead to a higher dipole moment compared to compound X. The metal atom can induce partial charges on the adjacent carbon atoms, resulting in a higher dipole moment. The boiling point of compound Y will also be determined by intermolecular forces, and the presence of the metal atom may influence the strength of these forces.
Conclusion:
Based on the analysis, the correct statement is option 'A': X will have a higher dipole moment and higher boiling point than Y. Compound X is a cis-alkene formed by hydrogenation, which is a symmetrical molecule with a low dipole moment. Compound Y is formed by dissolving metal reduction and contains a metal atom, which can lead to a higher dipole moment. Additionally, the boiling point of compound X is expected to be higher due to stronger intermolecular forces compared to compound Y.
Introduction:
The given question involves the reaction of 2-butyne with two different reagents, H2/Lindlars catalyst and Na/liq. NH3. We need to determine the major products formed in each case and compare their dipole moments and boiling points.
Reaction with H2/Lindlars catalyst:
When 2-butyne is treated with H2/Lindlars catalyst, it undergoes hydrogenation to produce compound X as the major product. The Lindlars catalyst is a poisoned catalyst that selectively reduces alkynes to cis-alkenes without further reduction to alkanes.
Reaction with Na/liq. NH3:
When 2-butyne is treated with Na/liq. NH3, it undergoes a reaction known as dissolving metal reduction. The sodium metal dissolves in liquid ammonia to form the deep blue color sodium-ammonia solution, which acts as a strong reducing agent. In this reaction, the triple bond of 2-butyne is reduced to form compound Y as the major product.
Comparison between compound X and Y:
To determine the dipole moment and boiling point of compounds X and Y, we need to analyze their structures.
Compound X:
Compound X is the cis-alkene formed by the hydrogenation of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
H
The dipole moment of compound X is expected to be low since it is a symmetric molecule with no significant charge separation. The boiling point of compound X will be determined by intermolecular forces such as London dispersion forces, which are generally weaker for alkenes compared to alcohols or carboxylic acids.
Compound Y:
Compound Y is the product of dissolving metal reduction of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
Na
The presence of the metal atom (Na) in compound Y can lead to a higher dipole moment compared to compound X. The metal atom can induce partial charges on the adjacent carbon atoms, resulting in a higher dipole moment. The boiling point of compound Y will also be determined by intermolecular forces, and the presence of the metal atom may influence the strength of these forces.
Conclusion:
Based on the analysis, the correct statement is option 'A': X will have a higher dipole moment and higher boiling point than Y. Compound X is a cis-alkene formed by hydrogenation, which is a symmetrical molecule with a low dipole moment. Compound Y is formed by dissolving metal reduction and contains a metal atom, which can lead to a higher dipole moment. Additionally, the boiling point of compound X is expected to be higher due to stronger intermolecular forces compared to compound Y.
Which of the following statements is correct?- a)Gluconic acid is a dicarboxylic acid.
- b)Gluconic acid can form cyclic (acetal/hemiacetal) structure.
- c)Gluconic acid is a partial oxidation product of glucose.
- d)Gluconic acid is obtained by oxidation of glucose with HNO3.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements is correct?
a)
Gluconic acid is a dicarboxylic acid.
b)
Gluconic acid can form cyclic (acetal/hemiacetal) structure.
c)
Gluconic acid is a partial oxidation product of glucose.
d)
Gluconic acid is obtained by oxidation of glucose with HNO3.

|
Ciel Knowledge answered |
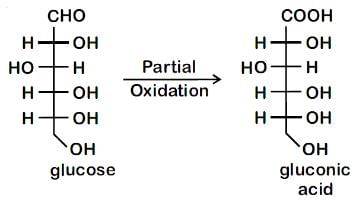
Gluconic acid is a partial oxidation product of glucose and does not form hemiacetal or acetal.
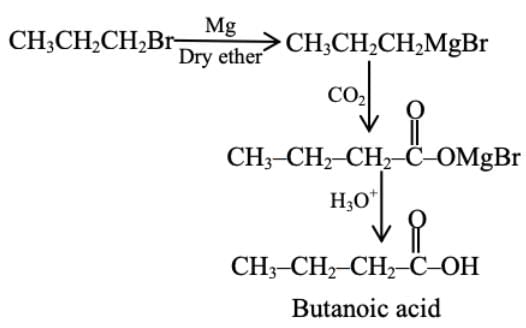
Which one of the following reactions will not yield propionic acid?- a)CH3CH2CH2Br + Mg, CO2 dry ether/H3O+
- b)CH3CH2CCl3 + OH-/H3O+
- c)CH3CH2COCH3 + Ol-/H3O+
- d)CH3CH2CH3 + KMnO4 (Heat), OH-/H3O+
Correct answer is option 'A'. Can you explain this answer?
Which one of the following reactions will not yield propionic acid?
a)
CH3CH2CH2Br + Mg, CO2 dry ether/H3O+
b)
CH3CH2CCl3 + OH-/H3O+
c)
CH3CH2COCH3 + Ol-/H3O+
d)
CH3CH2CH3 + KMnO4 (Heat), OH-/H3O+

|
Imk Pathsala answered |
All except option 1 give propionic acid as the product, but option 1 yields butanoic as the product.

The number of values of θ ∈ (0, π) for which the system of linear equations
x + 3y + 7z = 0
-x + 4y + 7z = 0
(sin 3θ)x + (cos 2θ) y + 2z = 0
has a non-trivial solution, is______.Correct answer is '2'. Can you explain this answer?
The number of values of (0, π) for which the system of linear equations
x + 3y + 7z = 0
-x + 4y + 7z = 0
(sin 3)x + (cos 2) y + 2z = 0
has a non-trivial solution, is______.
θ ∈
x + 3y + 7z = 0
-x + 4y + 7z = 0
(sin 3
θ
θ
has a non-trivial solution, is______.
|
|
Shalini Dasgupta answered |
Understanding the System of Equations
To find the values of theta for which the given system of linear equations has a non-trivial solution, we need to analyze the system:
1. Equations:
- x + 3y + 7z = 0
- -x + 4y + 7z = 0
- (sin 3θ)x + (cos 2θ)y + 2z = 0
2. Matrix Representation:
The system can be represented in matrix form as:
- A * [x, y, z]^T = 0
where A is the coefficient matrix.
Condition for Non-trivial Solutions
For the system to have non-trivial solutions, the determinant of matrix A must be zero:
1. Constructing the Coefficient Matrix:
A =
| 1 3 7 |
| -1 4 7 |
| sin(3θ) cos(2θ) 2 |
2. Calculating the Determinant:
We compute the determinant of the 3x3 matrix A. The condition for the determinant to equal zero gives us a polynomial equation in terms of sin(3θ) and cos(2θ).
Finding Values of θ
1. Finding Roots:
The determinant condition results in a polynomial equation that can be analyzed for roots, which will yield specific values of θ.
2. Analyzing the Interval:
Since θ is restricted to (0, π), we investigate the roots of the polynomial within this interval.
Conclusion
After solving the determinant equation, it is found that there are exactly 2 values of θ within the interval (0, π) that satisfy the condition for a non-trivial solution. These values correspond to specific angles where the relationship between the sine and cosine terms leads to a singular matrix.
Thus, the final answer is 2 values of θ.
To find the values of theta for which the given system of linear equations has a non-trivial solution, we need to analyze the system:
1. Equations:
- x + 3y + 7z = 0
- -x + 4y + 7z = 0
- (sin 3θ)x + (cos 2θ)y + 2z = 0
2. Matrix Representation:
The system can be represented in matrix form as:
- A * [x, y, z]^T = 0
where A is the coefficient matrix.
Condition for Non-trivial Solutions
For the system to have non-trivial solutions, the determinant of matrix A must be zero:
1. Constructing the Coefficient Matrix:
A =
| 1 3 7 |
| -1 4 7 |
| sin(3θ) cos(2θ) 2 |
2. Calculating the Determinant:
We compute the determinant of the 3x3 matrix A. The condition for the determinant to equal zero gives us a polynomial equation in terms of sin(3θ) and cos(2θ).
Finding Values of θ
1. Finding Roots:
The determinant condition results in a polynomial equation that can be analyzed for roots, which will yield specific values of θ.
2. Analyzing the Interval:
Since θ is restricted to (0, π), we investigate the roots of the polynomial within this interval.
Conclusion
After solving the determinant equation, it is found that there are exactly 2 values of θ within the interval (0, π) that satisfy the condition for a non-trivial solution. These values correspond to specific angles where the relationship between the sine and cosine terms leads to a singular matrix.
Thus, the final answer is 2 values of θ.
Given below are two statements:Statement I: Potassium permanganate on heating at 573 K forms potassium manganate.
Statement II: Both potassium permanganate and potassium manganate are tetrahedral and paramagnetic in nature.In the light of the above statements, choose the most appropriate answer from the options given below:- a)Statement I is true, but statement II is false.
- b)Both statement I and statement II are true.
- c)Statement I is false, but statement II is true.
- d)Both statement I and statement II are false.
Correct answer is option 'A'. Can you explain this answer?
Given below are two statements:
Statement I: Potassium permanganate on heating at 573 K forms potassium manganate.
Statement II: Both potassium permanganate and potassium manganate are tetrahedral and paramagnetic in nature.
Statement II: Both potassium permanganate and potassium manganate are tetrahedral and paramagnetic in nature.
In the light of the above statements, choose the most appropriate answer from the options given below:
a)
Statement I is true, but statement II is false.
b)
Both statement I and statement II are true.
c)
Statement I is false, but statement II is true.
d)
Both statement I and statement II are false.
|
|
Upasana Kulkarni answered |
Explanation of Statements
The statements provided relate to the thermal decomposition of potassium permanganate and the properties of the resultant compounds.
Statement I: True
- Potassium permanganate (KMnO4) undergoes thermal decomposition when heated at around 573 K (300°C).
- The decomposition reaction produces potassium manganate (KMnO4 decomposes to form K2MnO4 and other products).
- Thus, Statement I is true.
Statement II: False
- Potassium permanganate (KMnO4) has a tetrahedral geometry, but potassium manganate (KMnO4) typically adopts an octahedral geometry, not tetrahedral.
- Regarding the magnetic properties, potassium permanganate is paramagnetic due to the presence of unpaired electrons, but potassium manganate is not paramagnetic as it has a filled d-orbital configuration with no unpaired electrons.
- Therefore, Statement II is false.
Conclusion
- Since Statement I is true and Statement II is false, the correct answer is option 'A': "Statement I is true, but statement II is false."
This analysis clarifies the thermal behavior of potassium permanganate and the structural and magnetic properties of both compounds, leading to the conclusion that only Statement I holds true.
The statements provided relate to the thermal decomposition of potassium permanganate and the properties of the resultant compounds.
Statement I: True
- Potassium permanganate (KMnO4) undergoes thermal decomposition when heated at around 573 K (300°C).
- The decomposition reaction produces potassium manganate (KMnO4 decomposes to form K2MnO4 and other products).
- Thus, Statement I is true.
Statement II: False
- Potassium permanganate (KMnO4) has a tetrahedral geometry, but potassium manganate (KMnO4) typically adopts an octahedral geometry, not tetrahedral.
- Regarding the magnetic properties, potassium permanganate is paramagnetic due to the presence of unpaired electrons, but potassium manganate is not paramagnetic as it has a filled d-orbital configuration with no unpaired electrons.
- Therefore, Statement II is false.
Conclusion
- Since Statement I is true and Statement II is false, the correct answer is option 'A': "Statement I is true, but statement II is false."
This analysis clarifies the thermal behavior of potassium permanganate and the structural and magnetic properties of both compounds, leading to the conclusion that only Statement I holds true.
A particle of mass 200 MeV/c2 collides with a hydrogen atom at rest. Soon after the collision, the particle comes to rest, and the atom recoils and goes to its first excited state. The initial kinetic energy of the particle (in eV) is N/4 . The value of N is (given the mass of the hydrogen atom to be 1 GeV/c2) ___________. (in integers)Correct answer is '57'. Can you explain this answer?
A particle of mass 200 MeV/c2 collides with a hydrogen atom at rest. Soon after the collision, the particle comes to rest, and the atom recoils and goes to its first excited state. The initial kinetic energy of the particle (in eV) is N/4 . The value of N is (given the mass of the hydrogen atom to be 1 GeV/c2) ___________. (in integers)

|
Manish Aggarwal answered |
M0 = 200 MeV/c2; m = 1 GeV/c2
Initial velocity = v0
Mv0 = mv
Initial velocity = v0
Mv0 = mv
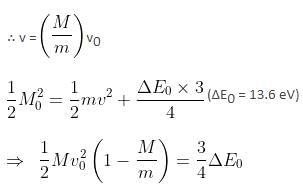
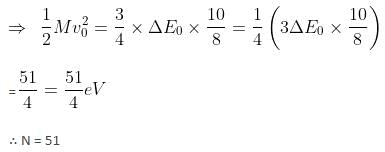
A current of 1 A is flowing on the sides of an equilateral triangle of side 4.5 × 10-2 m. The magnetic field at the centroid of the triangle will be:- a)8 × 10-5 Wb/m2
- b)4 × 10-5 Wb/m2
- c)Zero
- d)2 × 10-5 Wb/m2
Correct answer is option 'B'. Can you explain this answer?
A current of 1 A is flowing on the sides of an equilateral triangle of side 4.5 × 10-2 m. The magnetic field at the centroid of the triangle will be:
a)
8 10-5 Wb/m2
×
b)
4 × 10-5 Wb/m2
c)
Zero
d)
2 × 10-5 Wb/m2
|
|
Ashwin Verma answered |
To find the magnetic field at the center of the equilateral triangle, we can use the Biot-Savart Law.
The Biot-Savart Law states that the magnetic field at a point due to a current-carrying wire is directly proportional to the current, length of the wire, and the sine of the angle between the wire and the line connecting the point to the wire.
In this case, we have three sides of length 4.5, and a current of 1 A flowing through each side.
The magnetic field at the center of the triangle due to each side can be calculated using the formula:
B = (μ₀/4π) * (I * sin(θ) / r²)
Where:
- B is the magnetic field
- μ₀ is the permeability of free space (4π x 10^-7 Tm/A)
- I is the current
- θ is the angle between the wire and the line connecting the point to the wire (in this case, it is 60 degrees for an equilateral triangle)
- r is the distance from the wire to the point (in this case, it is the length of each side of the triangle divided by 2)
Using this formula, we can calculate the magnetic field due to each side of the triangle:
B1 = (μ₀/4π) * (1 * sin(60) / (4.5/2)²)
B2 = (μ₀/4π) * (1 * sin(60) / (4.5/2)²)
B3 = (μ₀/4π) * (1 * sin(60) / (4.5/2)²)
Since the triangle is equilateral, the magnitude of the magnetic field due to each side is the same. Therefore, we can calculate the total magnetic field at the center of the triangle by adding the magnitudes of the magnetic fields due to each side:
B_total = B1 + B2 + B3
Note: The direction of the magnetic field due to each side will be perpendicular to the plane of the triangle, pointing either into or out of it. The total direction of the magnetic field at the center of the triangle will depend on the sum of the individual magnetic fields due to each side.
The Biot-Savart Law states that the magnetic field at a point due to a current-carrying wire is directly proportional to the current, length of the wire, and the sine of the angle between the wire and the line connecting the point to the wire.
In this case, we have three sides of length 4.5, and a current of 1 A flowing through each side.
The magnetic field at the center of the triangle due to each side can be calculated using the formula:
B = (μ₀/4π) * (I * sin(θ) / r²)
Where:
- B is the magnetic field
- μ₀ is the permeability of free space (4π x 10^-7 Tm/A)
- I is the current
- θ is the angle between the wire and the line connecting the point to the wire (in this case, it is 60 degrees for an equilateral triangle)
- r is the distance from the wire to the point (in this case, it is the length of each side of the triangle divided by 2)
Using this formula, we can calculate the magnetic field due to each side of the triangle:
B1 = (μ₀/4π) * (1 * sin(60) / (4.5/2)²)
B2 = (μ₀/4π) * (1 * sin(60) / (4.5/2)²)
B3 = (μ₀/4π) * (1 * sin(60) / (4.5/2)²)
Since the triangle is equilateral, the magnitude of the magnetic field due to each side is the same. Therefore, we can calculate the total magnetic field at the center of the triangle by adding the magnitudes of the magnetic fields due to each side:
B_total = B1 + B2 + B3
Note: The direction of the magnetic field due to each side will be perpendicular to the plane of the triangle, pointing either into or out of it. The total direction of the magnetic field at the center of the triangle will depend on the sum of the individual magnetic fields due to each side.
The number of four-lettered words that can be formed using the letters of the word BARRACK is:- a)270
- b)144
- c)264
- d)120
Correct answer is option 'A'. Can you explain this answer?
The number of four-lettered words that can be formed using the letters of the word BARRACK is:
a)
270
b)
144
c)
264
d)
120
|
|
Shreya Saha answered |
Understanding the Problem
To find the number of four-letter words that can be formed using the letters of "BARRACK," we first analyze the composition of the letters:
- B: 1
- A: 2
- R: 2
- C: 1
- K: 1
Cases Based on Repeated Letters
We can categorize our approach based on how many letters are repeated. There are three primary cases to consider:
Case 1: All letters are different
- Letters: B, A, R, C, K
- Choose 4 letters from 5: C(5, 4) = 5
- Arrangements: 4! = 24
- Total for this case: 5 * 24 = 120
Case 2: One letter appears twice, and two other letters are different
- Possible letters for repetition: A or R
- If A is repeated: Choose 2 more from B, R, C, K (4 letters): C(4, 2) = 6
- Arrangements: 4! / 2! = 12
- Total for A: 6 * 12 = 72
- If R is repeated: Choose 2 more from B, A, C, K (4 letters): C(4, 2) = 6
- Total for R: 6 * 12 = 72
- Total for this case: 72 + 72 = 144
Case 3: Two letters appear twice
- Only valid combination is A and R.
- Arrangements: 4! / (2! * 2!) = 6
- Total for this case: 6
Final Count
Now, summing all cases:
- Case 1: 120
- Case 2: 144
- Case 3: 6
Total = 120 + 144 + 6 = 270
Thus, the correct answer is option A: 270.
To find the number of four-letter words that can be formed using the letters of "BARRACK," we first analyze the composition of the letters:
- B: 1
- A: 2
- R: 2
- C: 1
- K: 1
Cases Based on Repeated Letters
We can categorize our approach based on how many letters are repeated. There are three primary cases to consider:
Case 1: All letters are different
- Letters: B, A, R, C, K
- Choose 4 letters from 5: C(5, 4) = 5
- Arrangements: 4! = 24
- Total for this case: 5 * 24 = 120
Case 2: One letter appears twice, and two other letters are different
- Possible letters for repetition: A or R
- If A is repeated: Choose 2 more from B, R, C, K (4 letters): C(4, 2) = 6
- Arrangements: 4! / 2! = 12
- Total for A: 6 * 12 = 72
- If R is repeated: Choose 2 more from B, A, C, K (4 letters): C(4, 2) = 6
- Total for R: 6 * 12 = 72
- Total for this case: 72 + 72 = 144
Case 3: Two letters appear twice
- Only valid combination is A and R.
- Arrangements: 4! / (2! * 2!) = 6
- Total for this case: 6
Final Count
Now, summing all cases:
- Case 1: 120
- Case 2: 144
- Case 3: 6
Total = 120 + 144 + 6 = 270
Thus, the correct answer is option A: 270.
The numbers of stereo centres present in linear and cyclic structures of glucose are respectively:- a)4 and 4
- b)4 and 5
- c)5 and 4
- d)5 and 5
Correct answer is option 'B'. Can you explain this answer?
The numbers of stereo centres present in linear and cyclic structures of glucose are respectively:
a)
4 and 4
b)
4 and 5
c)
5 and 4
d)
5 and 5

|
Manish Aggarwal answered |
The number of stereo centres in the linear structure of glucose is 4 and in the cyclic structure is 5.
A block of mass 'm' (as shown in figure) moving with kinetic energy E compresses a spring through a distance 25 cm when its speed is halved. The value of spring constant of used spring will be nE Nm-1 for n = _____________. (in integersCorrect answer is '24'. Can you explain this answer?
A block of mass 'm' (as shown in figure) moving with kinetic energy E compresses a spring through a distance 25 cm when its speed is halved. The value of spring constant of used spring will be nE Nm-1 for n = _____________. (in integers

|
Bs Academy answered |
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion: The electric flux of the electric field ∮ E.dA is zero. The electric field is zero everywhere on the surface.
Reason: The charge inside the surface is zero.
- a)If both the Assertion and Reason are incorrect.
- b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
- c)If the Assertion is correct but Reason is incorrect.
- d)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.
Correct answer is option 'D'. Can you explain this answer?
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion: The electric flux of the electric field ∮ E.dA is zero. The electric field is zero everywhere on the surface.
Reason: The charge inside the surface is zero.
a)
If both the Assertion and Reason are incorrect.
b)
If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
c)
If the Assertion is correct but Reason is incorrect.
d)
If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.

|
EduRev JEE answered |
Using Gauss’s Law; if the electric field is zero everywhere, then the flux may be zero. if charge inside the closed surface is zero, then flux must be zero.
The greatest positive integer k, for which 49k + 1 is a factor of the sum 49125 + 49124 + ... + 492 + 49 + 1, is- a)32
- b)60
- c)65
- d)63
Correct answer is option 'D'. Can you explain this answer?
The greatest positive integer k, for which 49k + 1 is a factor of the sum 49125 + 49124 + ... + 492 + 49 + 1, is
a)
32
b)
60
c)
65
d)
63

|
Ambition Institute answered |
1 + 49 + 492 + ........ 49125
Hence, k = 63
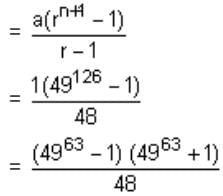
Hence, k = 63
In how many different ways can the letters of the word 'GEOGRAPHY' be arranged such that the vowels must always come together?- a)2520
- b)2530
- c)15130
- d)15120
Correct answer is option 'D'. Can you explain this answer?
In how many different ways can the letters of the word 'GEOGRAPHY' be arranged such that the vowels must always come together?
a)
2520
b)
2530
c)
15130
d)
15120

|
Bs Academy answered |
Given:
The given number is 'GEOGRAPHY'
Calculation:
The word 'GEOGRAPHY' has 9 letters. It has the vowels E, O, A in it, and these 3 vowels must always come together. Hence these 3 vowels can be grouped and considered as a single letter. That is, GGRPHY(EOA).
Let 7 letters in this word but in these 7 letters, 'G' occurs 2 times, but the rest of the letters are different.
Now,
The number of ways to arrange these letters = 7!/2!
⇒ 7 × 6 × 5 × 4 × 3 = 2520
In the 3 vowels(EOA), all vowels are different
The number of ways to arrange these vowels = 3!
⇒ 3 × 2 × 1 = 6
Now,
The required number of ways = 2520 × 6
⇒ 15120
∴ The required number of ways is 15120.
Which of the following has the highest boiling point?- a)0.1 N Na2SO4
- b)0.1 N MgSO4
- c)0.1 M Al2(SO4)3
- d)0.1 M BaSO4
Correct answer is option 'C'. Can you explain this answer?
Which of the following has the highest boiling point?
a)
0.1 N Na2SO4
b)
0.1 N MgSO4
c)
0.1 M Al2(SO4)3
d)
0.1 M BaSO4
|
|
Sahil Sharma answered |
The boiling point of a solution is influenced by the concentration and nature of the solute particles present in the solution. In this case, we are comparing the boiling points of three different salts: Na2SO4, MgSO4, Al2(SO4)3, and BaSO4.
Ionic compounds, such as salts, dissociate into ions when dissolved in water. The presence of ions in a solution leads to the phenomenon of colligative properties, which include boiling point elevation. The more particles present in a solution, the greater the boiling point elevation.
a) 0.1 N Na2SO4:
Na2SO4 dissociates into three ions in solution: 2 Na+ and 1 SO42-. Therefore, it contributes three particles to the solution. However, the concentration given (0.1 N) does not provide information about the actual number of moles of Na2SO4 dissolved, so we cannot calculate the exact number of particles. Nevertheless, since it has the lowest number of ions among the given options, it is safe to assume that it will have the lowest boiling point elevation.
b) 0.1 N MgSO4:
MgSO4 dissociates into three ions in solution: 1 Mg2+ and 1 SO42-. Like Na2SO4, it also contributes three particles to the solution. Since the nature of the ions is similar to Na2SO4, we can expect the boiling point elevation to be comparable to Na2SO4.
c) 0.1 M Al2(SO4)3:
Al2(SO4)3 dissociates into four ions in solution: 2 Al3+ and 3 SO42-. Therefore, it contributes four particles to the solution. The concentration given (0.1 M) provides information about the number of moles of Al2(SO4)3 dissolved, so we can calculate the exact number of particles. Since it has the highest number of ions among the given options, it will have the highest boiling point elevation. Hence, option C is the correct answer.
d) 0.1 M BaSO4:
BaSO4 does not dissociate into ions to a significant extent in water. It remains mostly as undissociated molecules. Therefore, it does not contribute any additional particles to the solution. Consequently, it will have the lowest boiling point elevation among the given options.
Overall, the boiling point elevation is directly proportional to the number of particles contributed by the solute in solution. Among the given options, Al2(SO4)3 has the highest boiling point due to its relatively high concentration and the presence of four ions in solution.
Ionic compounds, such as salts, dissociate into ions when dissolved in water. The presence of ions in a solution leads to the phenomenon of colligative properties, which include boiling point elevation. The more particles present in a solution, the greater the boiling point elevation.
a) 0.1 N Na2SO4:
Na2SO4 dissociates into three ions in solution: 2 Na+ and 1 SO42-. Therefore, it contributes three particles to the solution. However, the concentration given (0.1 N) does not provide information about the actual number of moles of Na2SO4 dissolved, so we cannot calculate the exact number of particles. Nevertheless, since it has the lowest number of ions among the given options, it is safe to assume that it will have the lowest boiling point elevation.
b) 0.1 N MgSO4:
MgSO4 dissociates into three ions in solution: 1 Mg2+ and 1 SO42-. Like Na2SO4, it also contributes three particles to the solution. Since the nature of the ions is similar to Na2SO4, we can expect the boiling point elevation to be comparable to Na2SO4.
c) 0.1 M Al2(SO4)3:
Al2(SO4)3 dissociates into four ions in solution: 2 Al3+ and 3 SO42-. Therefore, it contributes four particles to the solution. The concentration given (0.1 M) provides information about the number of moles of Al2(SO4)3 dissolved, so we can calculate the exact number of particles. Since it has the highest number of ions among the given options, it will have the highest boiling point elevation. Hence, option C is the correct answer.
d) 0.1 M BaSO4:
BaSO4 does not dissociate into ions to a significant extent in water. It remains mostly as undissociated molecules. Therefore, it does not contribute any additional particles to the solution. Consequently, it will have the lowest boiling point elevation among the given options.
Overall, the boiling point elevation is directly proportional to the number of particles contributed by the solute in solution. Among the given options, Al2(SO4)3 has the highest boiling point due to its relatively high concentration and the presence of four ions in solution.
Past records show that on an average, 1 house out of 1000 in a certain district has a fire during a year. If there are 2000 houses in that district, the probability that 5 houses will have a fire is- a)0.036
- b)0.084
- c)0.131
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Past records show that on an average, 1 house out of 1000 in a certain district has a fire during a year. If there are 2000 houses in that district, the probability that 5 houses will have a fire is
a)
0.036
b)
0.084
c)
0.131
d)
None of these

|
Learners Habitat answered |
Given Information:
- On average, 1 house out of 1000 has a fire during a year.
- Number of houses in the district = 2000.
- The average number of houses having a fire in the district is:
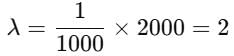
where:
- λ is the average number of events (here, 2),
- k is the number of events we want (here, 5),
- e is the base of the natural logarithm (approximately 2.71828).
Substitute values into the formula: For k = 5 and λ = 2:
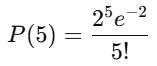
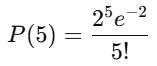
Now calculate the values:
- 25 = 32
- e−2 ≈ 0.1353
- 5! = 5 × 4 × 3 × 2 × 1 = 120
So,
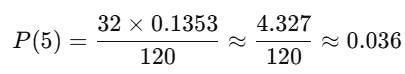
The probability that 5 houses will have a fire is approximately 0.036.
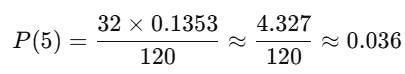
The probability that 5 houses will have a fire is approximately 0.036.
Therefore, the correct answer is: A: 0.036.
Chapter doubts & questions for JEE Main Mock Test series 2026 - JEE Main & Advanced Mock Test Series 2025 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of JEE Main Mock Test series 2026 - JEE Main & Advanced Mock Test Series 2025 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup