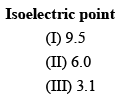All Exams >
Chemistry >
Organic Chemistry >
All Questions
All questions of Natural Products Chemistry for Chemistry Exam
The most basic amino acid among the following is:- a)Tyrosine
- b)Methionine
- c)Arginine
- d)Glutamine
Correct answer is option 'C'. Can you explain this answer?
The most basic amino acid among the following is:
a)
Tyrosine
b)
Methionine
c)
Arginine
d)
Glutamine

|
Kunal Goyal answered |
Basic Amino Acid: Arginine
Amino acids are the building blocks of proteins. There are 20 different types of amino acids commonly found in proteins. Out of these, some amino acids are basic, some are acidic, and some are neutral.
Basic amino acids are those that have a basic or alkaline side chain in their chemical structure. These amino acids have a positive charge at neutral pH (pH 7) due to the presence of an amino group (-NH2) in their structure.
Among the given options, the most basic amino acid is Arginine.
Explanation:
Arginine has a guanidine group (-NH-C(NH2)-NH2) in its side chain, which makes it a basic amino acid. This group has a pKa value of 12.5, which means that at neutral pH, the guanidine group will be mostly protonated (+NH3) and have a positive charge.
Methionine has a sulfur-containing side chain, but it is not basic in nature. Tyrosine has a hydroxyl group (-OH) in its side chain, which is not basic either. Glutamine has a neutral side chain and is not basic.
Hence, among the given options, Arginine is the most basic amino acid.
Amino acids are the building blocks of proteins. There are 20 different types of amino acids commonly found in proteins. Out of these, some amino acids are basic, some are acidic, and some are neutral.
Basic amino acids are those that have a basic or alkaline side chain in their chemical structure. These amino acids have a positive charge at neutral pH (pH 7) due to the presence of an amino group (-NH2) in their structure.
Among the given options, the most basic amino acid is Arginine.
Explanation:
Arginine has a guanidine group (-NH-C(NH2)-NH2) in its side chain, which makes it a basic amino acid. This group has a pKa value of 12.5, which means that at neutral pH, the guanidine group will be mostly protonated (+NH3) and have a positive charge.
Methionine has a sulfur-containing side chain, but it is not basic in nature. Tyrosine has a hydroxyl group (-OH) in its side chain, which is not basic either. Glutamine has a neutral side chain and is not basic.
Hence, among the given options, Arginine is the most basic amino acid.
How many of the following are reducing sugar:Sucrose, Lactose, Maltose, Glucose, Galactose, Fructose, Glycogen, Ribose.
Correct answer is '7'. Can you explain this answer?
How many of the following are reducing sugar:
Sucrose, Lactose, Maltose, Glucose, Galactose, Fructose, Glycogen, Ribose.
|
|
Pooja Choudhury answered |
The aldehyde group of aldoses is very susceptible to oxidation, whereas ketoses are less so, but can easily be oxidized if, like fructose, they contain an α-hydroxyl and can tautomerize to an aldose. Most monosaccharides are reducing sugars. This includes all of the common ones galactose, glucose, fructose, ribose, xylose, and mannose. Some disaccharides, such as lactose and maltose are reducing sugars since they have at least one anomeric carbon free, allowing that part of the sugar to linearize and yield an aldose.
The pKa values for the three ionizable groups X, Y and Z or Glutamic acid are 4.3, 9.7 and 2.2 respectively: The isoelectric point for the amino acid is:
The isoelectric point for the amino acid is: - a)7.00
- b)3.25
- c)4.95
- d)5.95
Correct answer is option 'B'. Can you explain this answer?
The pKa values for the three ionizable groups X, Y and Z or Glutamic acid are 4.3, 9.7 and 2.2 respectively:

The isoelectric point for the amino acid is:
a)
7.00
b)
3.25
c)
4.95
d)
5.95
|
|
Sandeep Sahoo answered |
Isoelectric point is calculated by the average of Pka values of left right side group.
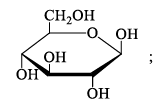
D-glucose and D-galactose are:
- a)C2-epimers
- b)C3-epimers
- c)C4-epimers
- d)Diastereomers
Correct answer is option 'C'. Can you explain this answer?
D-glucose and D-galactose are:
a)
C2-epimers
b)
C3-epimers
c)
C4-epimers
d)
Diastereomers
|
|
Vedika Singh answered |
Epimers are carbohydrates which vary in one position for the placement of the -OH group. The best examples are for D-glucose and D-galactose. Both monosaccharides are D-sugars, meaning that the -OH group on carbon-5 of these hexoses is located on the right in Fischer Projection.
Which of the following pairs give posit ive Tollen’s test:- a)Glucose, Sucrose
- b)Glucose, Fructose
- c)Hexanal, acetophenone
- d)Fructose, Sucrose
Correct answer is option 'B'. Can you explain this answer?
Which of the following pairs give posit ive Tollen’s test:
a)
Glucose, Sucrose
b)
Glucose, Fructose
c)
Hexanal, acetophenone
d)
Fructose, Sucrose

|
Ameya Rane answered |
It seems that the sentence is incomplete as it does not provide the options or pairs to choose from. Can you please provide the pairs so I can assist you further?
In a double stranded DNA, if the sequence 5’AGATCC3’ appears on one strand of DNA, what sequence appears on the complementary strand?- a)5’AGATCC3’
- b)5’CCTAGA3’
- c)5’GGATCT3’
- d)5’TCTAGG3’
Correct answer is option 'D'. Can you explain this answer?
In a double stranded DNA, if the sequence 5’AGATCC3’ appears on one strand of DNA, what sequence appears on the complementary strand?
a)
5’AGATCC3’
b)
5’CCTAGA3’
c)
5’GGATCT3’
d)
5’TCTAGG3’

|
Harshitha Sharma answered |
The complementary sequence to 5'-ATCGGCTA-3' is 3'-TAGCCGAT-5'. This is because in DNA, adenine (A) always pairs with thymine (T), and cytosine (C) always pairs with guanine (G). Therefore, the complementary strand will have T instead of A, G instead of C, C instead of G, and A instead of T in the corresponding positions.
Among the following, the incorrect statement(s) is/are:- a)Guanine is a Purine nucleobase
- b)Glycine and proline are achiral amino acids
- c)DNA contains glycosidic bonds and pentose sugars
- d)Sucrose is a non-reducing sugar.
Correct answer is option 'B'. Can you explain this answer?
Among the following, the incorrect statement(s) is/are:
a)
Guanine is a Purine nucleobase
b)
Glycine and proline are achiral amino acids
c)
DNA contains glycosidic bonds and pentose sugars
d)
Sucrose is a non-reducing sugar.

|
Priya Saha answered |
Incorrect Statements
Glycine and proline are achiral amino acids.
Explanation
Achiral molecules are those that do not have a mirror image that is different from the original molecule. Glycine and proline are not achiral amino acids.
Glycine is the simplest amino acid and has a hydrogen atom as its side chain (R-group). It is not chiral because it has two identical hydrogen atoms as its R-group.
Proline, on the other hand, has a unique structure due to the presence of a cyclic side chain that forms a five-membered ring. This ring structure causes proline to be a chiral molecule.
Therefore, statement B is incorrect.
Correct Statements
A) Guanine is a Purine nucleobase
B) DNA contains glycosidic bonds and pentose sugars
C) Sucrose is a non-reducing sugar.
Conclusion
In summary, glycine and proline are not achiral amino acids, making statement B incorrect. The other statements are correct. Guanine is a purine nucleobase, DNA contains glycosidic bonds and pentose sugars, and sucrose is a non-reducing sugar.
Glycine and proline are achiral amino acids.
Explanation
Achiral molecules are those that do not have a mirror image that is different from the original molecule. Glycine and proline are not achiral amino acids.
Glycine is the simplest amino acid and has a hydrogen atom as its side chain (R-group). It is not chiral because it has two identical hydrogen atoms as its R-group.
Proline, on the other hand, has a unique structure due to the presence of a cyclic side chain that forms a five-membered ring. This ring structure causes proline to be a chiral molecule.
Therefore, statement B is incorrect.
Correct Statements
A) Guanine is a Purine nucleobase
B) DNA contains glycosidic bonds and pentose sugars
C) Sucrose is a non-reducing sugar.
Conclusion
In summary, glycine and proline are not achiral amino acids, making statement B incorrect. The other statements are correct. Guanine is a purine nucleobase, DNA contains glycosidic bonds and pentose sugars, and sucrose is a non-reducing sugar.
The decreasing order of isoelectric point for the following a-amino acids is:
(I) Lysine (II) Alanine (III) Glutamic acid- a)II > I > III
- b)I > II > III
- c)III > I > II
- d)I > III > II
Correct answer is option 'B'. Can you explain this answer?
The decreasing order of isoelectric point for the following a-amino acids is:
(I) Lysine (II) Alanine (III) Glutamic acid
(I) Lysine (II) Alanine (III) Glutamic acid
a)
II > I > III
b)
I > II > III
c)
III > I > II
d)
I > III > II

|
Ipsita Chopra answered |
The decreasing order of isoelectric point for the given amino acids is:
(I) Lysine > (III) Glutamic acid > (II) Alanine
Lysine has a higher isoelectric point due to its positively charged side chain, which attracts negatively charged ions and increases the overall charge of the molecule at its isoelectric point. Glutamic acid has a lower isoelectric point than lysine, but still higher than alanine, due to its negatively charged side chain. Alanine has a neutral side chain and therefore has the lowest isoelectric point of the three amino acids.
(I) Lysine > (III) Glutamic acid > (II) Alanine
Lysine has a higher isoelectric point due to its positively charged side chain, which attracts negatively charged ions and increases the overall charge of the molecule at its isoelectric point. Glutamic acid has a lower isoelectric point than lysine, but still higher than alanine, due to its negatively charged side chain. Alanine has a neutral side chain and therefore has the lowest isoelectric point of the three amino acids.
D-glucose and D-fructose can be distinguished by:- a)Ph—NH—NH2
- b)[Ag(NH3)2]OH
- c)Br2/H2O
- d)NaOH/Cu2+
Correct answer is option 'C'. Can you explain this answer?
D-glucose and D-fructose can be distinguished by:
a)
Ph—NH—NH2
b)
[Ag(NH3)2]OH
c)
Br2/H2O
d)
NaOH/Cu2+

|
Uma Bharti answered |
Fructose contain keto group while glucose contsin aldehyde grp option c is mildoxidosing agnt convert aldo groip only
The pH value of a solution in which a polar amino acid does not migrate under the influence of electric field is called:- a)Isoelectric po int
- b)Isoelectronic point
- c)Neutralizat ion point
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
The pH value of a solution in which a polar amino acid does not migrate under the influence of electric field is called:
a)
Isoelectric po int
b)
Isoelectronic point
c)
Neutralizat ion point
d)
None of these

|
Ishaan Sengupta answered |
The pH value of a solution in which a polar amino acid does not migrate under the influence of an electric field is called the isoelectric point.
Explanation:
Polar Amino Acids:
Amino acids are organic compounds that contain both an amino group (-NH2) and a carboxyl group (-COOH). They are the building blocks of proteins. Some amino acids have polar side chains, which means they have a partial positive or negative charge.
Electric Field and Migration:
When an electric field is applied to a solution containing charged particles, such as polar amino acids, the charged particles will migrate towards the oppositely charged electrode. The migration of charged particles in an electric field is called electrophoresis.
Isoelectric Point:
The isoelectric point (pI) is the pH value at which a polar amino acid does not migrate under the influence of an electric field. At this pH, the net charge on the amino acid is zero, and it remains stationary in the electric field.
Charge of Amino Acids:
The charge on an amino acid depends on the pH of the solution. At low pH values (acidic conditions), the amino acid is protonated and carries a positive charge. At high pH values (basic conditions), the amino acid is deprotonated and carries a negative charge. At the isoelectric point, the amino acid is in its zwitterionic form, which means it has both a positive and a negative charge that cancel each other out, resulting in a net charge of zero.
pH and Isoelectric Point:
The pH at which the isoelectric point occurs depends on the specific amino acid. Each amino acid has a unique isoelectric point based on the properties of its side chain. For example, the isoelectric point of lysine is around pH 9.8, while the isoelectric point of glutamic acid is around pH 3.2.
Conclusion:
The pH value at which a polar amino acid does not migrate under the influence of an electric field is called the isoelectric point. At this pH, the amino acid is in its zwitterionic form and carries no net charge.
The complementary strand of DNA for the following single stranded DNA sequence: 5' - A - T - C- A - T - G - C-3' is:- a)5' - A - T - C- A - T - G - C-3'
- b)5' - T - A - G - T - A - C- G -3'
- c)5' - G - C- A - T - G - A - T -3'
- d)5' - C- G - T - A - C- T - A -3'
Correct answer is option 'C'. Can you explain this answer?
The complementary strand of DNA for the following single stranded DNA sequence: 5' - A - T - C- A - T - G - C-3' is:
a)
5' - A - T - C- A - T - G - C-3'
b)
5' - T - A - G - T - A - C- G -3'
c)
5' - G - C- A - T - G - A - T -3'
d)
5' - C- G - T - A - C- T - A -3'
|
|
Vedika Singh answered |
Correct Answer :- C
Explanation : In DNA, Adenine (A) pairs with Thymine (T) and Guanine (G) pairs with Cytosine (C).
Hence, on replication of DNA in M phase, complementary nucleotides for each base pair will be added as follows:
A - T
T - A
G - C
C - G
Since replication occurs in 5'-3' direction, so the complementary strand will be formed in 3'-5' direction.
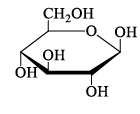
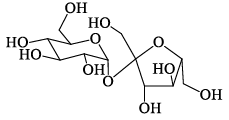
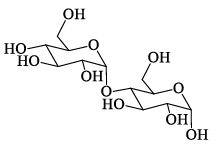
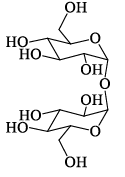
A disaccharide does not give a posit ive test for Tollen’s reagent. Upon acidic hydrolysis, it gives an equimolar mixture of two different monosaccharides, both the which can be oxidized by bromine water. This disaccharide is:- a)
- b)
- c)
- d)
Correct answer is option 'D'. Can you explain this answer?
A disaccharide does not give a posit ive test for Tollen’s reagent. Upon acidic hydrolysis, it gives an equimolar mixture of two different monosaccharides, both the which can be oxidized by bromine water. This disaccharide is:
a)

b)

c)

d)

|
|
Subha Som answered |
D is ketal which doesn't break in aq sol
Which of the following pairs is/are correctly matched:- a)Galactose-Lactose
- b)Fructose-Glucose
- c)Fructose-Galactose
- d)Ribose-Deoyribose
Correct answer is option 'B,C,D'. Can you explain this answer?
Which of the following pairs is/are correctly matched:
a)
Galactose-Lactose
b)
Fructose-Glucose
c)
Fructose-Galactose
d)
Ribose-Deoyribose
|
|
Aditya Deshmukh answered |
(b) Cassini–Huygens is an unmanned spacecraft sent to the planet Saturn. Therefore, option 1 is wrong, this eliminates option (a), (c) and (d). Now we are left with final answer B only 2 and 3. MESSENGER is a robotic NASA spacecraft orbiting the planet Mercury. Voyager 1 (September 1977) and voyager 2 (Aug 1977) were launched to study the outer Solar System
D-Glucose exist in x different forms. The value of x(stereoisomer) is:
Correct answer is '3'. Can you explain this answer?
D-Glucose exist in x different forms. The value of x(stereoisomer) is:

|
Pranavi Mishra answered |
**D-Glucose Stereoisomers**
D-Glucose is a monosaccharide, a type of carbohydrate, and it exists in different forms known as stereoisomers. Stereoisomers are compounds that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. In the case of D-Glucose, the arrangement of its hydroxyl groups around the asymmetric carbon atoms gives rise to different stereoisomers.
**Definition of Stereoisomers**
Stereoisomers can be categorized into two main types: enantiomers and diastereomers.
1. **Enantiomers**: Enantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other. They have the same connectivity but differ in their three-dimensional orientation. Enantiomers are optically active, meaning they rotate plane-polarized light in opposite directions. In the case of D-Glucose, there are two enantiomers: D-Glucose and L-Glucose.
2. **Diastereomers**: Diastereomers are stereoisomers that are not mirror images of each other. They have different connectivity or spatial arrangement at one or more stereocenters. Diastereomers are not optically active. In the case of D-Glucose, there are three diastereomers: α-D-Glucose, β-D-Glucose, and open-chain D-Glucose.
**Explanation of the Answer: 3**
The question asks for the number of different forms (stereoisomers) in which D-Glucose exists. The correct answer is '3'. Here's the breakdown of the three stereoisomers:
1. **Enantiomers (1 stereoisomer)**:
- D-Glucose (Dextrose): It is the naturally occurring form of glucose found in living organisms.
- L-Glucose: It is the mirror image of D-Glucose and is not commonly found in nature.
2. **Diastereomers (2 stereoisomers)**:
- α-D-Glucose: It is a cyclic form of glucose where the hydroxyl group on the anomeric carbon is in the axial position.
- β-D-Glucose: It is another cyclic form of glucose where the hydroxyl group on the anomeric carbon is in the equatorial position.
Therefore, considering both enantiomers and diastereomers, D-Glucose exists in a total of 3 different forms or stereoisomers.
D-Glucose is a monosaccharide, a type of carbohydrate, and it exists in different forms known as stereoisomers. Stereoisomers are compounds that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. In the case of D-Glucose, the arrangement of its hydroxyl groups around the asymmetric carbon atoms gives rise to different stereoisomers.
**Definition of Stereoisomers**
Stereoisomers can be categorized into two main types: enantiomers and diastereomers.
1. **Enantiomers**: Enantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other. They have the same connectivity but differ in their three-dimensional orientation. Enantiomers are optically active, meaning they rotate plane-polarized light in opposite directions. In the case of D-Glucose, there are two enantiomers: D-Glucose and L-Glucose.
2. **Diastereomers**: Diastereomers are stereoisomers that are not mirror images of each other. They have different connectivity or spatial arrangement at one or more stereocenters. Diastereomers are not optically active. In the case of D-Glucose, there are three diastereomers: α-D-Glucose, β-D-Glucose, and open-chain D-Glucose.
**Explanation of the Answer: 3**
The question asks for the number of different forms (stereoisomers) in which D-Glucose exists. The correct answer is '3'. Here's the breakdown of the three stereoisomers:
1. **Enantiomers (1 stereoisomer)**:
- D-Glucose (Dextrose): It is the naturally occurring form of glucose found in living organisms.
- L-Glucose: It is the mirror image of D-Glucose and is not commonly found in nature.
2. **Diastereomers (2 stereoisomers)**:
- α-D-Glucose: It is a cyclic form of glucose where the hydroxyl group on the anomeric carbon is in the axial position.
- β-D-Glucose: It is another cyclic form of glucose where the hydroxyl group on the anomeric carbon is in the equatorial position.
Therefore, considering both enantiomers and diastereomers, D-Glucose exists in a total of 3 different forms or stereoisomers.
Two forms of D-glucopyranose, are called:- a)Enantiomers
- b)Diastereomers
- c)Epimers
- d)Anomers
Correct answer is option 'D'. Can you explain this answer?
Two forms of D-glucopyranose, are called:
a)
Enantiomers
b)
Diastereomers
c)
Epimers
d)
Anomers
|
|
Kalpana Pandey answered |
Because, the configuration is changed only at anomeric carbon and rest are same...
Which gives red colour with Fehling’s solut ion:- a)Glucose
- b)Cellulose
- c)Benzaldehyde
- d)Cane sugar
Correct answer is option 'A'. Can you explain this answer?
Which gives red colour with Fehling’s solut ion:
a)
Glucose
b)
Cellulose
c)
Benzaldehyde
d)
Cane sugar
|
|
Sandeep Sahoo answered |
All monosacharides are reducing sugar. Glucose is a monosacharide and reducing sugar. Reducing sugars reacts with feeling's solutions, benedict's solutions and tollens reangets. So glucose gives red colour with fehling solutions.
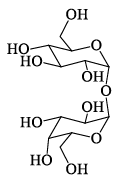
Among α- and β-anomeric pairs which one is more stable: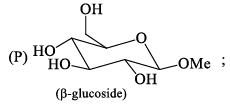
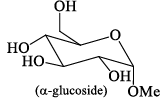
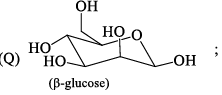
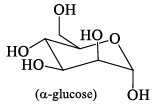
- a)P-β; Q- β
- b)P-β; Q- α
- c)P-α; Q- α
- d)P-α; Q- β
Correct answer is option 'D'. Can you explain this answer?
Among α- and β-anomeric pairs which one is more stable:




a)
P-β; Q- β
b)
P-β; Q- α
c)
P-α; Q- α
d)
P-α; Q- β

|
Saurabh Chavan answered |
Minimum number of -oH and -CH2OH group on axial position gets more stability. In Q of 1st only group on axial and in 2nd 2 groups on axial.
A nannopeptide contains ………….. pepetide linkages:- a)10
- b)8
- c)9
- d)18
Correct answer is option 'B'. Can you explain this answer?
A nannopeptide contains ………….. pepetide linkages:
a)
10
b)
8
c)
9
d)
18

|
Milan Majumdar answered |
Ans.
Option (b)
The peptide bond is formed between two amino acids by the elimination of a water molecule. A dipeptide contains one peptide linkage. A tripeptide contains two peptide linkages. Similarly, a nanopeptide contains 8 peptide linkages.
One sequence of amino acids respects for long distances in silk protein. Complete hydrolysis of one mole of a fragment with this sequence gives 2 mol alanine, 3 mol glycine, and 1 mol serine. Partial hydrolysis yields Ala-Gly-Ala, Gly-Ala-Gly, Gly-Ser-Gly, and Ser-Gly-Ala peptides. What is the amino acid repeat:- a)Gly-Gly-Ser-Ala-Gly-Ala
- b)Gly-Gly-Gly-Ala-Ala-Ser
- c)Ser-Ala-Ala-Gly-Gly-Gly
- d)Gly-Ser-Gly-Ala-Gly-Ala
Correct answer is option 'B'. Can you explain this answer?
One sequence of amino acids respects for long distances in silk protein. Complete hydrolysis of one mole of a fragment with this sequence gives 2 mol alanine, 3 mol glycine, and 1 mol serine. Partial hydrolysis yields Ala-Gly-Ala, Gly-Ala-Gly, Gly-Ser-Gly, and Ser-Gly-Ala peptides. What is the amino acid repeat:
a)
Gly-Gly-Ser-Ala-Gly-Ala
b)
Gly-Gly-Gly-Ala-Ala-Ser
c)
Ser-Ala-Ala-Gly-Gly-Gly
d)
Gly-Ser-Gly-Ala-Gly-Ala

|
Dipanjan Sharma answered |
Explanation:
- The given information states that one mole of a fragment with a specific sequence of amino acids gives 2 mol alanine, 3 mol glycine, and 1 mol serine upon complete hydrolysis.
- Partial hydrolysis yields four peptides: Ala-Gly-Ala, Gly-Ala-Gly, Gly-Ser-Gly, and Ser-Gly-Ala.
- It can be observed that Gly occurs the most frequently in the partial hydrolysis peptides, and it also occurs the most frequently in the complete hydrolysis product.
- Therefore, the amino acid repeat should start with Gly.
- The sequence of partial hydrolysis peptides can be used to determine the order of the amino acids in the repeat.
- The partial hydrolysis peptides are Gly-Ala-Gly, Ala-Gly-Ala, Gly-Ser-Gly, and Ser-Gly-Ala.
- The repeat should be a sequence that includes all of these peptides, in the order they are given.
- Gly occurs twice in a row in the first partial hydrolysis peptide, so the repeat should have two consecutive Gly.
- Ala occurs before and after Gly in the first and second partial hydrolysis peptides, respectively, so the repeat should have two consecutive Ala.
- Ser occurs before and after Gly in the third and fourth partial hydrolysis peptides, respectively, so the repeat should have Ser in between two Gly.
- Therefore, the amino acid repeat is Gly-Gly-Ala-Ala-Gly-Gly.
- Option B is the correct answer.
Which one among the following is a Sesquiterpene:- a)
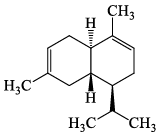
- b)
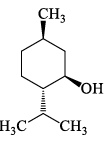
- c)
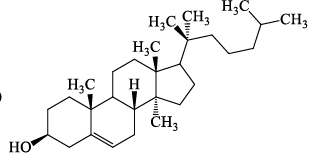
- d)
Correct answer is option 'A'. Can you explain this answer?
Which one among the following is a Sesquiterpene:
a)

b)

c)

d)

|
|
Madhu Bala answered |
Total number of carbon atoms= 15
Therefore the number of isopreane units =3
it is sesquiterpene.
Therefore the number of isopreane units =3
it is sesquiterpene.
Which one is not a constituent of nuclei acid:- a)Uracil
- b)Guanidine
- c)Phosphoric acid
- d)Ribose sugar
Correct answer is option 'B'. Can you explain this answer?
Which one is not a constituent of nuclei acid:
a)
Uracil
b)
Guanidine
c)
Phosphoric acid
d)
Ribose sugar

|
Anirban Khanna answered |
A constituent of nucleic acid are uracil, phosphorid acid, ribose sugar and deoxyribose sugar. So, guanidine is not a constituent of nucleic acid.
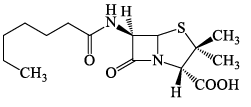
The following carbohydrate is: 
- a)A ketohexose
- b)An aldohexose
- c)A β-pyranose
- d)An α-pyranose
Correct answer is option 'B,C'. Can you explain this answer?
The following carbohydrate is: 

a)
A ketohexose
b)
An aldohexose
c)
A β-pyranose
d)
An α-pyranose
|
|
Suraj Srj answered |
Aldohexose - four asymmetric centers, an aldehyde group, six carbon atoms and five alcoholic group in Fischer projection.
Pyran stands for six membered ring (one hetroatom included).
Alpha- pyranose if OH group at C-1 is axial downward.
Beta- pyranose if OH group at C-1 is equatorial upward.
Therefore, above given cyclic hemiacetal structure is an aldohexose and a beta- pyranose.
Which of the following compounds will form an osazone derivative?- a)CH3CH2COCH2OH
- b)CH3COCH2CH2OH
- c)CH3CH2CHOHCH2OH
- d) CH3CH2COCH2OCH3
Correct answer is option 'A'. Can you explain this answer?
Which of the following compounds will form an osazone derivative?
a)
CH3CH2COCH2OH
b)
CH3COCH2CH2OH
c)
CH3CH2CHOHCH2OH
d)
CH3CH2COCH2OCH3

|
Ishaan Sengupta answered |
When sugars are reacted with excess of phenylhydrazine osazone formation takes place.
Osazone will form in that sugar only where keto hydroxy group is present.
Thus CH3CH2COCH2OH will form an osazone derivative; as it has keto hydroxy group.
The structure of D-(+)-glucose is: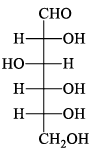 The structure of L-(–)-glucose is:
The structure of L-(–)-glucose is:- a)
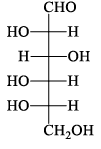
- b)
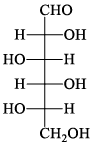
- c)
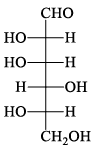
- d)
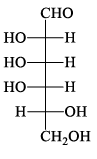
Correct answer is option 'A'. Can you explain this answer?
The structure of D-(+)-glucose is:

The structure of L-(–)-glucose is:
a)

b)

c)

d)


|
Yashdeep Maurya answered |
Just take the mirror image of the give D+ glucose ..you will see that option A will be seen....you just imagine a mirror is on that right side of the D glucose and now image the structure which would be seen in mirror...that's it
In fructose the possible optical iso mer are:
Correct answer is '8'. Can you explain this answer?
In fructose the possible optical iso mer are:

|
Pragati Sharma answered |
Fructose is a monosaccharide or simple sugar that is found in many fruits, vegetables and honey. It has the chemical formula C6H12O6 and is an important source of energy for the body. When fructose is dissolved in water, it can exist in different forms or isomers, depending on the spatial arrangement of its atoms.
Possible Optical Isomers of Fructose
Fructose has four chiral carbon atoms, which means that it can have up to 2^4 or 16 possible stereoisomers. However, not all of these isomers are optically active or have the ability to rotate plane-polarized light. Only those isomers that are non-superimposable mirror images of each other are optically active and are called enantiomers or optical isomers.
Fructose exists in two main forms: alpha fructose and beta fructose. These two forms differ in the orientation of their hydroxyl groups around the anomeric carbon atom, which is the carbon atom that is bonded to both an oxygen atom and another carbon atom. Alpha fructose has its hydroxyl group pointing downwards, while beta fructose has its hydroxyl group pointing upwards.
Each of these forms can exist in two possible configurations, depending on the orientation of the hydroxyl groups around the other three chiral carbon atoms. These configurations are designated as either D or L, depending on the orientation of the hydroxyl group on the last chiral carbon atom. Therefore, fructose can exist in four possible isomers: alpha-D-fructose, alpha-L-fructose, beta-D-fructose, and beta-L-fructose.
Each of these four isomers can exist in two possible enantiomeric forms, depending on whether the molecule is a right-handed or left-handed form. Therefore, fructose can exist in eight possible optical isomers: alpha-D-fructose, alpha-L-fructose, beta-D-fructose, beta-L-fructose, (alpha-D)-fructose, (alpha-L)-fructose, (beta-D)-fructose, and (beta-L)-fructose.
Conclusion
In summary, fructose has eight possible optical isomers, which are determined by the orientation of its hydroxyl groups around its four chiral carbon atoms. These isomers are important for understanding the biological activity and metabolism of fructose, as well as for the production of pure fructose for use in food and pharmaceutical applications.
Possible Optical Isomers of Fructose
Fructose has four chiral carbon atoms, which means that it can have up to 2^4 or 16 possible stereoisomers. However, not all of these isomers are optically active or have the ability to rotate plane-polarized light. Only those isomers that are non-superimposable mirror images of each other are optically active and are called enantiomers or optical isomers.
Fructose exists in two main forms: alpha fructose and beta fructose. These two forms differ in the orientation of their hydroxyl groups around the anomeric carbon atom, which is the carbon atom that is bonded to both an oxygen atom and another carbon atom. Alpha fructose has its hydroxyl group pointing downwards, while beta fructose has its hydroxyl group pointing upwards.
Each of these forms can exist in two possible configurations, depending on the orientation of the hydroxyl groups around the other three chiral carbon atoms. These configurations are designated as either D or L, depending on the orientation of the hydroxyl group on the last chiral carbon atom. Therefore, fructose can exist in four possible isomers: alpha-D-fructose, alpha-L-fructose, beta-D-fructose, and beta-L-fructose.
Each of these four isomers can exist in two possible enantiomeric forms, depending on whether the molecule is a right-handed or left-handed form. Therefore, fructose can exist in eight possible optical isomers: alpha-D-fructose, alpha-L-fructose, beta-D-fructose, beta-L-fructose, (alpha-D)-fructose, (alpha-L)-fructose, (beta-D)-fructose, and (beta-L)-fructose.
Conclusion
In summary, fructose has eight possible optical isomers, which are determined by the orientation of its hydroxyl groups around its four chiral carbon atoms. These isomers are important for understanding the biological activity and metabolism of fructose, as well as for the production of pure fructose for use in food and pharmaceutical applications.
How many different tripeptides can be obtained from alanine, glycine and phenylalanine, each tripeptide containing all the three amino acids?
Correct answer is '6'. Can you explain this answer?
How many different tripeptides can be obtained from alanine, glycine and phenylalanine, each tripeptide containing all the three amino acids?

|
Arnab Pillai answered |
Introduction:
In this question, we need to determine the number of different tripeptides that can be obtained from the amino acids alanine, glycine, and phenylalanine. Each tripeptide must contain all three amino acids.
Explanation:
To solve this problem, we need to consider the different arrangements of the three amino acids in a tripeptide. Since each tripeptide must contain all three amino acids, we can use the concept of permutations to find the number of different tripeptides.
Permutations:
Permutations are arrangements of objects where the order matters. In this case, the order of the amino acids in the tripeptide matters.
Using the formula for permutations:
The number of permutations of n objects taken r at a time is given by the formula:
P(n, r) = n! / (n - r)!
Applying the formula:
In this case, we have 3 amino acids (alanine, glycine, and phenylalanine) that need to be arranged in tripeptides. Since each tripeptide contains exactly 3 amino acids, we can use the formula for permutations with n = 3 and r = 3.
P(3, 3) = 3! / (3 - 3)!
= 3! / 0!
The factorial of 3 is 3! = 3 * 2 * 1 = 6.
The factorial of 0 is defined as 1.
Therefore, the calculation simplifies to:
P(3, 3) = 6 / 1 = 6
Conclusion:
Hence, there are 6 different tripeptides that can be obtained from the amino acids alanine, glycine, and phenylalanine, where each tripeptide contains all three amino acids.
In this question, we need to determine the number of different tripeptides that can be obtained from the amino acids alanine, glycine, and phenylalanine. Each tripeptide must contain all three amino acids.
Explanation:
To solve this problem, we need to consider the different arrangements of the three amino acids in a tripeptide. Since each tripeptide must contain all three amino acids, we can use the concept of permutations to find the number of different tripeptides.
Permutations:
Permutations are arrangements of objects where the order matters. In this case, the order of the amino acids in the tripeptide matters.
Using the formula for permutations:
The number of permutations of n objects taken r at a time is given by the formula:
P(n, r) = n! / (n - r)!
Applying the formula:
In this case, we have 3 amino acids (alanine, glycine, and phenylalanine) that need to be arranged in tripeptides. Since each tripeptide contains exactly 3 amino acids, we can use the formula for permutations with n = 3 and r = 3.
P(3, 3) = 3! / (3 - 3)!
= 3! / 0!
The factorial of 3 is 3! = 3 * 2 * 1 = 6.
The factorial of 0 is defined as 1.
Therefore, the calculation simplifies to:
P(3, 3) = 6 / 1 = 6
Conclusion:
Hence, there are 6 different tripeptides that can be obtained from the amino acids alanine, glycine, and phenylalanine, where each tripeptide contains all three amino acids.
Synthesis of each molecule of glucose in photosynthesis involves:- a)18 molecules of ATP
- b)10 molecules of ATP
- c)8 molecules of ATP
- d)6 molecules of ATP
Correct answer is option 'A'. Can you explain this answer?
Synthesis of each molecule of glucose in photosynthesis involves:
a)
18 molecules of ATP
b)
10 molecules of ATP
c)
8 molecules of ATP
d)
6 molecules of ATP

|
Saikat Ghoshal answered |
Overview:
The synthesis of each molecule of glucose in photosynthesis involves the utilization of ATP (adenosine triphosphate) as an energy source. ATP is a high-energy molecule that provides the necessary energy for various cellular processes, including glucose synthesis. In the process of photosynthesis, plants convert sunlight, water, and carbon dioxide into glucose and oxygen. The synthesis of glucose occurs through a series of complex reactions known as the Calvin cycle.
Explanation:
The Calvin cycle is the set of reactions that occur in the stroma of chloroplasts during photosynthesis. It involves a series of enzyme-catalyzed reactions that utilize ATP and NADPH (nicotinamide adenine dinucleotide phosphate) generated during the light-dependent reactions. The primary purpose of the Calvin cycle is the fixation of carbon dioxide and the synthesis of carbohydrates, particularly glucose.
Steps involved in glucose synthesis:
1. Carbon fixation:
- In the first step of the Calvin cycle, carbon dioxide molecules are incorporated into an organic molecule called ribulose-1,5-bisphosphate (RuBP).
- This reaction is catalyzed by the enzyme ribulose bisphosphate carboxylase/oxygenase (Rubisco).
- This process results in the formation of two molecules of 3-phosphoglycerate (3-PGA).
2. Reduction:
- The second step involves the conversion of 3-PGA into glyceraldehyde-3-phosphate (G3P).
- This conversion requires ATP and NADPH as energy and reducing power sources, respectively.
- Each molecule of 3-PGA is phosphorylated by ATP and reduced by NADPH to form G3P.
3. Regeneration:
- In the final step, G3P molecules are used to regenerate RuBP, which is necessary for the continuation of the Calvin cycle.
- Several ATP molecules are utilized in this step to convert G3P into RuBP.
ATP utilization in glucose synthesis:
- During the Calvin cycle, a total of 18 molecules of ATP are utilized in the synthesis of each molecule of glucose.
- ATP is involved in various steps of the cycle, including the phosphorylation of 3-PGA to form G3P and the regeneration of RuBP.
- The energy provided by ATP is essential for driving the endergonic reactions of glucose synthesis.
Therefore, the correct answer is option 'A' - 18 molecules of ATP.
The synthesis of each molecule of glucose in photosynthesis involves the utilization of ATP (adenosine triphosphate) as an energy source. ATP is a high-energy molecule that provides the necessary energy for various cellular processes, including glucose synthesis. In the process of photosynthesis, plants convert sunlight, water, and carbon dioxide into glucose and oxygen. The synthesis of glucose occurs through a series of complex reactions known as the Calvin cycle.
Explanation:
The Calvin cycle is the set of reactions that occur in the stroma of chloroplasts during photosynthesis. It involves a series of enzyme-catalyzed reactions that utilize ATP and NADPH (nicotinamide adenine dinucleotide phosphate) generated during the light-dependent reactions. The primary purpose of the Calvin cycle is the fixation of carbon dioxide and the synthesis of carbohydrates, particularly glucose.
Steps involved in glucose synthesis:
1. Carbon fixation:
- In the first step of the Calvin cycle, carbon dioxide molecules are incorporated into an organic molecule called ribulose-1,5-bisphosphate (RuBP).
- This reaction is catalyzed by the enzyme ribulose bisphosphate carboxylase/oxygenase (Rubisco).
- This process results in the formation of two molecules of 3-phosphoglycerate (3-PGA).
2. Reduction:
- The second step involves the conversion of 3-PGA into glyceraldehyde-3-phosphate (G3P).
- This conversion requires ATP and NADPH as energy and reducing power sources, respectively.
- Each molecule of 3-PGA is phosphorylated by ATP and reduced by NADPH to form G3P.
3. Regeneration:
- In the final step, G3P molecules are used to regenerate RuBP, which is necessary for the continuation of the Calvin cycle.
- Several ATP molecules are utilized in this step to convert G3P into RuBP.
ATP utilization in glucose synthesis:
- During the Calvin cycle, a total of 18 molecules of ATP are utilized in the synthesis of each molecule of glucose.
- ATP is involved in various steps of the cycle, including the phosphorylation of 3-PGA to form G3P and the regeneration of RuBP.
- The energy provided by ATP is essential for driving the endergonic reactions of glucose synthesis.
Therefore, the correct answer is option 'A' - 18 molecules of ATP.
From a carboxymethyl-cellulose column at pH 6.0, Arginine, valine and Glutamic acid will elute in the order:- a)Arginine, valine, Glutamic acid
- b)Arginine, Glutamic acid, valine
- c)Glutamic acid, Arginine, valine
- d)Glutamic acid, valine, Arginine
Correct answer is option 'D'. Can you explain this answer?
From a carboxymethyl-cellulose column at pH 6.0, Arginine, valine and Glutamic acid will elute in the order:
a)
Arginine, valine, Glutamic acid
b)
Arginine, Glutamic acid, valine
c)
Glutamic acid, Arginine, valine
d)
Glutamic acid, valine, Arginine

|
Aryan Choudhary answered |
Elution order of Arginine, Valine, and Glutamic acid from carboxymethyl-cellulose column at pH 6.0
Carboxymethyl-cellulose (CMC) column is a type of ion exchange chromatography in which anion exchange resins interact with positively charged molecules. The elution order of amino acids from CMC column depends on their pKa values and the pH of the buffer used. At pH 6.0, the carboxyl groups on CMC resin are partially ionized, and the amino acids are in their zwitterionic form.
The pKa values of Arginine, Valine, and Glutamic acid are as follows:
- Arginine: pKa1=2.17, pKa2=9.04, pKa3=12.48
- Valine: pKa1=2.32, pKa2=9.62
- Glutamic acid: pKa1=2.19, pKa2=4.25, pKa3=9.67
Based on the pKa values and the pH of the buffer, the following elution order is expected:
- Glutamic acid (pI=3.22) will elute first because it has the lowest pI value among the three amino acids and will be negatively charged in the column.
- Valine (pI=6.01) will elute next because it is neutral at pH 6.0 and does not interact strongly with the CMC resin.
- Arginine (pI=10.76) will elute last because it has the highest pI value and will be positively charged in the column, interacting strongly with the negatively charged CMC resin.
Therefore, the correct elution order of Arginine, Valine, and Glutamic acid from CMC column at pH 6.0 is:
- Glutamic acid, Valine, Arginine
Carboxymethyl-cellulose (CMC) column is a type of ion exchange chromatography in which anion exchange resins interact with positively charged molecules. The elution order of amino acids from CMC column depends on their pKa values and the pH of the buffer used. At pH 6.0, the carboxyl groups on CMC resin are partially ionized, and the amino acids are in their zwitterionic form.
The pKa values of Arginine, Valine, and Glutamic acid are as follows:
- Arginine: pKa1=2.17, pKa2=9.04, pKa3=12.48
- Valine: pKa1=2.32, pKa2=9.62
- Glutamic acid: pKa1=2.19, pKa2=4.25, pKa3=9.67
Based on the pKa values and the pH of the buffer, the following elution order is expected:
- Glutamic acid (pI=3.22) will elute first because it has the lowest pI value among the three amino acids and will be negatively charged in the column.
- Valine (pI=6.01) will elute next because it is neutral at pH 6.0 and does not interact strongly with the CMC resin.
- Arginine (pI=10.76) will elute last because it has the highest pI value and will be positively charged in the column, interacting strongly with the negatively charged CMC resin.
Therefore, the correct elution order of Arginine, Valine, and Glutamic acid from CMC column at pH 6.0 is:
- Glutamic acid, Valine, Arginine
Which pair(s) of amino acids cannot have hydrophobic interactions:- a)Leucine and alanine
- b)Arginine and Glutamic acid
- c)Glycine and asparagine
- d)Glutamic acid and serine
Correct answer is option 'B,C,D'. Can you explain this answer?
Which pair(s) of amino acids cannot have hydrophobic interactions:
a)
Leucine and alanine
b)
Arginine and Glutamic acid
c)
Glycine and asparagine
d)
Glutamic acid and serine

|
Shilpa Datta answered |
Explanation:
Hydrophobic interactions refer to the tendency of nonpolar molecules to cluster together in an aqueous solution. Amino acids can be classified as hydrophobic or hydrophilic based on their polarity.
A) Leucine and alanine:
- Leucine is a hydrophobic amino acid with a nonpolar side chain.
- Alanine is also a hydrophobic amino acid with a nonpolar side chain.
- Therefore, these two amino acids can have hydrophobic interactions.
B) Arginine and glutamic acid:
- Arginine is a hydrophilic amino acid with a positively charged side chain.
- Glutamic acid is a hydrophilic amino acid with a negatively charged side chain.
- Since both amino acids are hydrophilic, they cannot have hydrophobic interactions.
C) Glycine and asparagine:
- Glycine is a hydrophilic amino acid with a small, nonpolar side chain.
- Asparagine is a hydrophilic amino acid with a polar side chain.
- Since both amino acids are hydrophilic, they cannot have hydrophobic interactions.
D) Glutamic acid and serine:
- Glutamic acid is a hydrophilic amino acid with a negatively charged side chain.
- Serine is a hydrophilic amino acid with a polar side chain.
- Since both amino acids are hydrophilic, they cannot have hydrophobic interactions.
Therefore, the correct answer is options B, C, and D.
Hydrophobic interactions refer to the tendency of nonpolar molecules to cluster together in an aqueous solution. Amino acids can be classified as hydrophobic or hydrophilic based on their polarity.
A) Leucine and alanine:
- Leucine is a hydrophobic amino acid with a nonpolar side chain.
- Alanine is also a hydrophobic amino acid with a nonpolar side chain.
- Therefore, these two amino acids can have hydrophobic interactions.
B) Arginine and glutamic acid:
- Arginine is a hydrophilic amino acid with a positively charged side chain.
- Glutamic acid is a hydrophilic amino acid with a negatively charged side chain.
- Since both amino acids are hydrophilic, they cannot have hydrophobic interactions.
C) Glycine and asparagine:
- Glycine is a hydrophilic amino acid with a small, nonpolar side chain.
- Asparagine is a hydrophilic amino acid with a polar side chain.
- Since both amino acids are hydrophilic, they cannot have hydrophobic interactions.
D) Glutamic acid and serine:
- Glutamic acid is a hydrophilic amino acid with a negatively charged side chain.
- Serine is a hydrophilic amino acid with a polar side chain.
- Since both amino acids are hydrophilic, they cannot have hydrophobic interactions.
Therefore, the correct answer is options B, C, and D.
The correct statement about the following disaccharide is: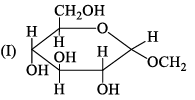
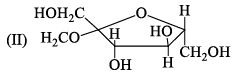
- a)Ring I is pyranose with α-glycosidic link
- b)Ring I is furanose with α-glycosidic link
- c)Ring II is furanose with α-glycosidic link
- d)Ring II is pyranose with β-glycosidic link
Correct answer is option 'A'. Can you explain this answer?
The correct statement about the following disaccharide is:


a)
Ring I is pyranose with α-glycosidic link
b)
Ring I is furanose with α-glycosidic link
c)
Ring II is furanose with α-glycosidic link
d)
Ring II is pyranose with β-glycosidic link

|
Sonu Choudhary answered |
As we know that ,the pyranose is 6 membered ring and furanose is 5 membered ring therfore otion A is correct ....
All natural amino acids, except one, react with cold nitrous acid and produce nitrogen gas. Which one of the following is that odd amino acid:- a)Tryptophan
- b)Glycine
- c)Proline
- d)Hist idine
Correct answer is option 'C'. Can you explain this answer?
All natural amino acids, except one, react with cold nitrous acid and produce nitrogen gas. Which one of the following is that odd amino acid:
a)
Tryptophan
b)
Glycine
c)
Proline
d)
Hist idine

|
Sinjini Nair answered |
Explanation:
Amino acids are organic compounds that contain both an amino group (-NH2) and a carboxyl group (-COOH). They are the building blocks of proteins and play an essential role in various biological processes. When amino acids react with cold nitrous acid (HNO2), they undergo a process known as nitrosation, where the amino group is replaced by a nitroso group (-NO).
All natural amino acids, except one, react with cold nitrous acid and produce nitrogen gas. This is due to the fact that the nitroso group (-NO) in nitrous acid reacts with the amino group (-NH2) in amino acids to form a diazonium salt, which then decomposes to form nitrogen gas (N2). However, one amino acid does not undergo this reaction, and that is proline.
The reason why proline is the odd amino acid is because of its unique structure. Proline is an imino acid, which means it has an additional nitrogen atom in its ring structure. This nitrogen atom is not a part of the amino group, and therefore, it cannot react with nitrous acid to form nitrogen gas. Instead, the nitroso group in nitrous acid reacts with the carboxyl group (-COOH) in proline to form a nitrite salt.
In summary, all natural amino acids, except proline, react with cold nitrous acid to produce nitrogen gas. Proline does not undergo this reaction due to its unique structure, which prevents the amino group from reacting with nitrous acid.
Amino acids are organic compounds that contain both an amino group (-NH2) and a carboxyl group (-COOH). They are the building blocks of proteins and play an essential role in various biological processes. When amino acids react with cold nitrous acid (HNO2), they undergo a process known as nitrosation, where the amino group is replaced by a nitroso group (-NO).
All natural amino acids, except one, react with cold nitrous acid and produce nitrogen gas. This is due to the fact that the nitroso group (-NO) in nitrous acid reacts with the amino group (-NH2) in amino acids to form a diazonium salt, which then decomposes to form nitrogen gas (N2). However, one amino acid does not undergo this reaction, and that is proline.
The reason why proline is the odd amino acid is because of its unique structure. Proline is an imino acid, which means it has an additional nitrogen atom in its ring structure. This nitrogen atom is not a part of the amino group, and therefore, it cannot react with nitrous acid to form nitrogen gas. Instead, the nitroso group in nitrous acid reacts with the carboxyl group (-COOH) in proline to form a nitrite salt.
In summary, all natural amino acids, except proline, react with cold nitrous acid to produce nitrogen gas. Proline does not undergo this reaction due to its unique structure, which prevents the amino group from reacting with nitrous acid.
Ident ify the correct set of stereochemical relat ionships amongst the following monosaccharides I-IV: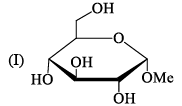
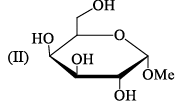
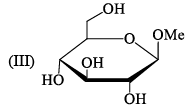
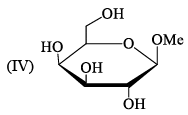
- a)I and II are anomers; III and IV are epimers
- b)I and III are epimers; II and IV are anomers
- c)I and II are epimers; III and IV are anomers
- d)I and III are anomers; I and II are epimers
Correct answer is option 'D'. Can you explain this answer?
Ident ify the correct set of stereochemical relat ionships amongst the following monosaccharides I-IV:




a)
I and II are anomers; III and IV are epimers
b)
I and III are epimers; II and IV are anomers
c)
I and II are epimers; III and IV are anomers
d)
I and III are anomers; I and II are epimers
|
|
Subha Som answered |
I, III are C2 epimer II,IV are C1 ie anomer
How many moles of formaldehyde will be formed: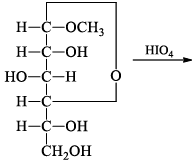
- a)1
- b)2
- c)3
- d)0
Correct answer is option 'A'. Can you explain this answer?
How many moles of formaldehyde will be formed:

a)
1
b)
2
c)
3
d)
0

|
Naman Gupta answered |
Only the 6th c arbon give the formaldehyde and from 5th carbon we get formic acid
Which of the following regarding to the examples of Keto sugar is/are correct:- a)Fructose
- b)Ribulose
- c)Ribose sugar
- d)Dihydroxy acetose
Correct answer is option 'A,B,D'. Can you explain this answer?
Which of the following regarding to the examples of Keto sugar is/are correct:
a)
Fructose
b)
Ribulose
c)
Ribose sugar
d)
Dihydroxy acetose
|
|
Biswa Nath answered |
Keto sugar means which contains >C=O groups.
Fructose, Ribulose, Dihydroxy acetose all of them contains >C=O group.
But, in Ribose sugar there have no such groups.
Fructose, Ribulose, Dihydroxy acetose all of them contains >C=O group.
But, in Ribose sugar there have no such groups.
There are 20 naturally occurring amino acids. The maximum number of tripeptides that can be obtained is:- a)6470
- b)7465
- c)8000
- d)5360
Correct answer is option 'C'. Can you explain this answer?
There are 20 naturally occurring amino acids. The maximum number of tripeptides that can be obtained is:
a)
6470
b)
7465
c)
8000
d)
5360

|
Swara Reddy answered |
Naturally occurring amino acids are 20. Hence, number of possible tripeptides
= 203 = 8000
Tollens test will be negative for:- a)Glucose
- b)Mannose
- c)Sucrose
- d)Galactose
Correct answer is option 'C'. Can you explain this answer?
Tollens test will be negative for:
a)
Glucose
b)
Mannose
c)
Sucrose
d)
Galactose

|
Niharika Kulkarni answered |
Tollens test and its principle:
Tollens test is a chemical test used to detect the presence of aldehydes in a given sample. It is also known as the silver mirror test. The test is based on the principle that aldehydes are easily oxidized to carboxylic acids and can be reduced to form silver mirror-like precipitates in the presence of Tollens reagent.
Negative Tollens test for Sucrose:
Sucrose is a disaccharide composed of glucose and fructose monomers. It is a non-reducing sugar, meaning that it does not have an aldehyde or ketone functional group that could be oxidized to form a silver mirror-like precipitate. Therefore, Tollens test will not give a positive result for sucrose.
Positive Tollens test for Glucose, Mannose and Galactose:
Glucose, Mannose, and Galactose are monosaccharides that have an aldehyde functional group capable of being oxidized by Tollens reagent. When these sugars are treated with Tollens reagent, they will undergo oxidation and reduce the Tollens reagent to form a silver mirror-like precipitate. Hence, Tollens test will give a positive result for glucose, mannose, and galactose.
Conclusion:
In conclusion, Tollens test is a chemical test used to detect the presence of aldehydes in a given sample. Sucrose is a non-reducing sugar, and it does not have an aldehyde functional group, so Tollens test will not give a positive result for sucrose. On the other hand, glucose, mannose, and galactose are reducing sugars, and they have an aldehyde functional group, so Tollens test will give a positive result for these sugars.
Tollens test is a chemical test used to detect the presence of aldehydes in a given sample. It is also known as the silver mirror test. The test is based on the principle that aldehydes are easily oxidized to carboxylic acids and can be reduced to form silver mirror-like precipitates in the presence of Tollens reagent.
Negative Tollens test for Sucrose:
Sucrose is a disaccharide composed of glucose and fructose monomers. It is a non-reducing sugar, meaning that it does not have an aldehyde or ketone functional group that could be oxidized to form a silver mirror-like precipitate. Therefore, Tollens test will not give a positive result for sucrose.
Positive Tollens test for Glucose, Mannose and Galactose:
Glucose, Mannose, and Galactose are monosaccharides that have an aldehyde functional group capable of being oxidized by Tollens reagent. When these sugars are treated with Tollens reagent, they will undergo oxidation and reduce the Tollens reagent to form a silver mirror-like precipitate. Hence, Tollens test will give a positive result for glucose, mannose, and galactose.
Conclusion:
In conclusion, Tollens test is a chemical test used to detect the presence of aldehydes in a given sample. Sucrose is a non-reducing sugar, and it does not have an aldehyde functional group, so Tollens test will not give a positive result for sucrose. On the other hand, glucose, mannose, and galactose are reducing sugars, and they have an aldehyde functional group, so Tollens test will give a positive result for these sugars.
Which one of the following compounds will form an osazone derivative:- a)CH3CH2CHOHCH2OH
- b)CH3COCH2CH2OH
- c)CH3CH2COCH2OH
- d)CH3CH2COCH2OCH3
Correct answer is option 'C'. Can you explain this answer?
Which one of the following compounds will form an osazone derivative:
a)
CH3CH2CHOHCH2OH
b)
CH3COCH2CH2OH
c)
CH3CH2COCH2OH
d)
CH3CH2COCH2OCH3

|
Sarthak Chavan answered |
When sugars are reacted with excess of phenylhydrazine osazone formation takes place.Osazone will form in that sugar only where keto hydroxy group is present.
Thus CH3CH2COCH2OH will form an osazone derivative; as it has keto hydroxy group.
Which of the following pairs give the same osazone?- a)D-glucose; D-mannose
- b)D-galactose; D-tallose
- c)D-gulose; D-idose
- d)D-altrose; D-glucose
Correct answer is option 'A,B,C'. Can you explain this answer?
Which of the following pairs give the same osazone?
a)
D-glucose; D-mannose
b)
D-galactose; D-tallose
c)
D-gulose; D-idose
d)
D-altrose; D-glucose

|
Niharika Kulkarni answered |
Pairing of Osazones:
Osazones are crystalline compounds that are formed by the reaction of reducing sugars with phenylhydrazine. They have characteristic structures that can be used to identify the sugar involved.
In this question, we are given four pairs of sugars and we need to determine which pairs give the same osazone.
Pair a) D-glucose; D-mannose:
D-glucose and D-mannose are both hexose sugars with the same formula, C6H12O6. They differ only in the configuration of one carbon atom. When these sugars are treated with phenylhydrazine, they will form the same osazone.
Pair b) D-galactose; D-tallose:
D-galactose and D-tallose are also hexose sugars, but they have different structures. When these sugars are treated with phenylhydrazine, they will form different osazones.
Pair c) D-gulose; D-idose:
D-gulose and D-idose are both hexose sugars, but they have different structures. When these sugars are treated with phenylhydrazine, they will form different osazones.
Pair d) D-altrose; D-glucose:
D-altrose and D-glucose are both hexose sugars, but they have different structures. When these sugars are treated with phenylhydrazine, they will form different osazones.
Conclusion:
From the given pairs, only pair a) D-glucose; D-mannose gives the same osazone. The other pairs, b) D-galactose; D-tallose and c) D-gulose; D-idose, give different osazones.
Therefore, the correct answer is option 'A, B, C'.
Osazones are crystalline compounds that are formed by the reaction of reducing sugars with phenylhydrazine. They have characteristic structures that can be used to identify the sugar involved.
In this question, we are given four pairs of sugars and we need to determine which pairs give the same osazone.
Pair a) D-glucose; D-mannose:
D-glucose and D-mannose are both hexose sugars with the same formula, C6H12O6. They differ only in the configuration of one carbon atom. When these sugars are treated with phenylhydrazine, they will form the same osazone.
Pair b) D-galactose; D-tallose:
D-galactose and D-tallose are also hexose sugars, but they have different structures. When these sugars are treated with phenylhydrazine, they will form different osazones.
Pair c) D-gulose; D-idose:
D-gulose and D-idose are both hexose sugars, but they have different structures. When these sugars are treated with phenylhydrazine, they will form different osazones.
Pair d) D-altrose; D-glucose:
D-altrose and D-glucose are both hexose sugars, but they have different structures. When these sugars are treated with phenylhydrazine, they will form different osazones.
Conclusion:
From the given pairs, only pair a) D-glucose; D-mannose gives the same osazone. The other pairs, b) D-galactose; D-tallose and c) D-gulose; D-idose, give different osazones.
Therefore, the correct answer is option 'A, B, C'.
An acidic amino acid among the following is:- a)Valine
- b)Proline
- c)Ananine
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
An acidic amino acid among the following is:
a)
Valine
b)
Proline
c)
Ananine
d)
None of these

|
Anshika Chavan answered |
The correct answer is option 'D' (None of these).
Explanation:
Acidity in amino acids is determined by the presence of carboxyl groups (-COOH) in their molecular structure. When the carboxyl group is ionized, it releases a hydrogen ion (H+) and forms a negatively charged carboxylate ion (-COO-), making the amino acid acidic in nature.
Among the options given:
a) Valine: Valine is a nonpolar, aliphatic amino acid and does not contain a carboxyl group. Therefore, it is not acidic.
b) Proline: Proline is an imino acid and does contain a carboxyl group. However, the nitrogen atom in proline's side chain can react with the carboxyl group, forming a cyclic structure called a pyrrolidine ring. This ring structure reduces the acidity of proline. Therefore, proline is not considered an acidic amino acid.
c) Alanine: There seems to be a spelling mistake in the option provided. The correct name of the amino acid is "Alanine." Alanine is a nonpolar, aliphatic amino acid and does not contain a carboxyl group. Therefore, it is not acidic.
Based on the above explanations, none of the options provided (Valine, Proline, Alanine) are acidic amino acids. Hence, the correct answer is option 'D' (None of these).
Explanation:
Acidity in amino acids is determined by the presence of carboxyl groups (-COOH) in their molecular structure. When the carboxyl group is ionized, it releases a hydrogen ion (H+) and forms a negatively charged carboxylate ion (-COO-), making the amino acid acidic in nature.
Among the options given:
a) Valine: Valine is a nonpolar, aliphatic amino acid and does not contain a carboxyl group. Therefore, it is not acidic.
b) Proline: Proline is an imino acid and does contain a carboxyl group. However, the nitrogen atom in proline's side chain can react with the carboxyl group, forming a cyclic structure called a pyrrolidine ring. This ring structure reduces the acidity of proline. Therefore, proline is not considered an acidic amino acid.
c) Alanine: There seems to be a spelling mistake in the option provided. The correct name of the amino acid is "Alanine." Alanine is a nonpolar, aliphatic amino acid and does not contain a carboxyl group. Therefore, it is not acidic.
Based on the above explanations, none of the options provided (Valine, Proline, Alanine) are acidic amino acids. Hence, the correct answer is option 'D' (None of these).
Biuret test is not given by:- a)Proteins
- b)Urea
- c)Polypeptide
- d)Carbohydrates
Correct answer is option 'D'. Can you explain this answer?
Biuret test is not given by:
a)
Proteins
b)
Urea
c)
Polypeptide
d)
Carbohydrates

|
Rajnee Kalyan answered |
Biuret test is used for analysis of peptide linkage (polypeptide and protein are such type of example)
reagent - cuso4+naoh/koh + na,k tartrate(roshelle salt)
how it work - copper add with the nitrogen form a complex which absorb wavelength at 544 nm and shows purple or yellow colour
main point - short length amino acid shows pink or blue colour , it analysis not only peptide bond but also peptide like bond (eg urea) , the test gives information about concentration of protein higher the con of protein higher will be the intensity of purple /pink/blue/yellow solutuon
reagent - cuso4+naoh/koh + na,k tartrate(roshelle salt)
how it work - copper add with the nitrogen form a complex which absorb wavelength at 544 nm and shows purple or yellow colour
main point - short length amino acid shows pink or blue colour , it analysis not only peptide bond but also peptide like bond (eg urea) , the test gives information about concentration of protein higher the con of protein higher will be the intensity of purple /pink/blue/yellow solutuon
The beta and alpha glucose have different specific rotations. When either is dissolved in water, their rotation changes until the same fixed value results. This is called:- a)Epimerisation
- b)Racemisation
- c)Anomerisation
- d)Mutarotation
Correct answer is option 'D'. Can you explain this answer?
The beta and alpha glucose have different specific rotations. When either is dissolved in water, their rotation changes until the same fixed value results. This is called:
a)
Epimerisation
b)
Racemisation
c)
Anomerisation
d)
Mutarotation

|
Uma Bharti answered |
When they are dissolved an equilibrium misture is obtained which has specific rotation value...so it doesnt matter whether it is alpha or beta form
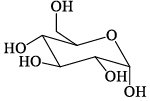
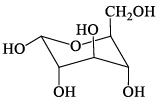
Among the D-glucopyranose forms given below, which is more stable:- a)
- b)
- c)
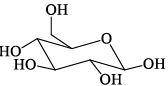
- d)
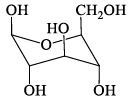
Correct answer is option 'C'. Can you explain this answer?
Among the D-glucopyranose forms given below, which is more stable:
a)

b)

c)

d)


|
Saurabh Chavan answered |
There should be 0 or minimum number of OH or CH2OH group on axial position hence then gives more stability.
The number of amino acids and number of peptides bonds in a linear tetrapeptide (made of different amino acids) are respectively:- a)4 and 4
- b)5 and 5
- c)5 and 4
- d)4 and 3
Correct answer is option 'D'. Can you explain this answer?
The number of amino acids and number of peptides bonds in a linear tetrapeptide (made of different amino acids) are respectively:
a)
4 and 4
b)
5 and 5
c)
5 and 4
d)
4 and 3

|
Nikhil Thakur answered |
@----@-----@-----@ consider @ as a amino acid and dotted lines as a peptide bond we clearly see that four amino acid have 3 bond .
Glucose and mannose are:- a)keto-hexoses
- b)Anomers
- c)Epimers
- d)Disaccharides
Correct answer is option 'C'. Can you explain this answer?
Glucose and mannose are:
a)
keto-hexoses
b)
Anomers
c)
Epimers
d)
Disaccharides
|
|
Avinash Mehta answered |
Epimers are compounds having the same chemical formula but differ in the spatial arrangement around a single carbon atom. D-Galactose, on the other hand, differs from D-Glucose ONLY at the 4th Carbon atom. Hence, it is a C-4 epimer of D-Glucose. D-Mannose and D-Galactose.
The number of disulphide linkage present in insulin are:- a)4
- b)3
- c)2
- d)1
Correct answer is option 'B'. Can you explain this answer?
The number of disulphide linkage present in insulin are:
a)
4
b)
3
c)
2
d)
1

|
Harshitha Sharma answered |
Insulin Structure and Disulphide Linkages
Insulin is a hormone produced by the pancreas that plays a crucial role in regulating blood sugar levels. It consists of two polypeptide chains, known as the A chain and the B chain, which are connected by disulfide linkages. These disulfide linkages are formed between specific cysteine residues in the chains and are essential for the structure and function of insulin.
Disulfide Linkages in Insulin
Insulin contains three disulfide linkages, which are responsible for stabilizing its three-dimensional structure. These linkages form between the sulfur atoms of cysteine residues, which are amino acids that contain a sulfur atom in their side chains.
The disulfide linkages in insulin are as follows:
1. A7-B7: This disulfide linkage connects the A chain and the B chain at position 7. It forms between the sulfur atom of cysteine residue 7 in the A chain and the sulfur atom of cysteine residue 7 in the B chain.
2. A20-B19: This disulfide linkage connects the A chain and the B chain at position 20 and 19, respectively. It forms between the sulfur atom of cysteine residue 20 in the A chain and the sulfur atom of cysteine residue 19 in the B chain.
3. A6-A11: This disulfide linkage connects two cysteine residues within the A chain. It forms between the sulfur atoms of cysteine residue 6 and cysteine residue 11.
These three disulfide linkages contribute to the folding and stability of insulin's tertiary structure. They help to maintain the correct spatial arrangement of the A and B chains, which is crucial for insulin's ability to bind to its receptor and exert its biological effects.
Conclusion
In conclusion, insulin contains three disulfide linkages that connect the A and B chains and contribute to its overall structure and function. These linkages are formed between specific cysteine residues and play a crucial role in the stability and activity of insulin. Therefore, the correct answer is option 'B' - insulin has three disulfide linkages.
Insulin is a hormone produced by the pancreas that plays a crucial role in regulating blood sugar levels. It consists of two polypeptide chains, known as the A chain and the B chain, which are connected by disulfide linkages. These disulfide linkages are formed between specific cysteine residues in the chains and are essential for the structure and function of insulin.
Disulfide Linkages in Insulin
Insulin contains three disulfide linkages, which are responsible for stabilizing its three-dimensional structure. These linkages form between the sulfur atoms of cysteine residues, which are amino acids that contain a sulfur atom in their side chains.
The disulfide linkages in insulin are as follows:
1. A7-B7: This disulfide linkage connects the A chain and the B chain at position 7. It forms between the sulfur atom of cysteine residue 7 in the A chain and the sulfur atom of cysteine residue 7 in the B chain.
2. A20-B19: This disulfide linkage connects the A chain and the B chain at position 20 and 19, respectively. It forms between the sulfur atom of cysteine residue 20 in the A chain and the sulfur atom of cysteine residue 19 in the B chain.
3. A6-A11: This disulfide linkage connects two cysteine residues within the A chain. It forms between the sulfur atoms of cysteine residue 6 and cysteine residue 11.
These three disulfide linkages contribute to the folding and stability of insulin's tertiary structure. They help to maintain the correct spatial arrangement of the A and B chains, which is crucial for insulin's ability to bind to its receptor and exert its biological effects.
Conclusion
In conclusion, insulin contains three disulfide linkages that connect the A and B chains and contribute to its overall structure and function. These linkages are formed between specific cysteine residues and play a crucial role in the stability and activity of insulin. Therefore, the correct answer is option 'B' - insulin has three disulfide linkages.
Chapter doubts & questions for Natural Products Chemistry - Organic Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Natural Products Chemistry - Organic Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Organic Chemistry
39 videos|92 docs|46 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup