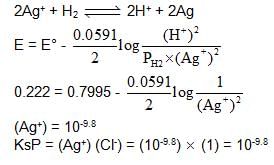KVS PGT Chemistry Mock Test - 3 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test KVS PGT Exam Mock Test Series 2025 - KVS PGT Chemistry Mock Test - 3
In the following question, an idiomatic expression and its four possible meanings are given. Find out the correct meaning of the idiom.
Q. A house divided against itself cannot stand.
Directions: In the following question, a sentence is given with a blank to be filled in with an appropriate word. Select the correct alternative out of the four and indicate it as your answer.
Kalidas, _______ wrote some fine dramas, is famous.
Kalidas, _______ wrote some fine dramas, is famous.
Directions: Improve the bracketed part of the sentence.
Q. No other Indian was a (great) orator than Sir Surendranath.
नीचे दिए गए शब्दों का सही संधि वाला विकल्प पहचानिए?
धर्म + अर्थ
The leadership can be executed only by a person who exhibits
One kg of carbon produces __________ kg of carbon dioxide.
During dehydration of alcohols to alkenes by heating with conc. H2SO4 the initiation step is-
[AIEEE-2003]
On moving down a group the number of valence electrons:
In a mixture of A and B, components show negative deviation when [AIEEE-2002]
The metal that can not be obtained by electrolysis of an aqueous solution of its salt is
[JEE Main 2014]
When a zinc rod is kept in a copper nitrate solution what happens?
At 87°C, the following equilibrium is established
H2(g) + S(s) 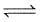 H2S(g) Kp = 7 × 10-2
H2S(g) Kp = 7 × 10-2
If 0.50 mole of hydrogen and 1.0 mole of sulfur are heated to 87°C in 1.0 L vessel, what will be the partial pressure of H2S at equilibrium ?
When a chemical bond is formed, there is decrease in
If Ksp for HgSO4 is 6.4 × 10-5, then solubility of this substance in mole per m3 is
Match the Column I with Column II and mark the correct option from the codes given below.
Phenyl magnesium bromide reacts with methanol to give -
[AIEEE 2006]
Following cell has EMF 0.7995 V.
Pt | H2 (1 atm) | HNO3 (1M) || AgNO3 (1M) | Ag
If we add enough KCl to the Ag cell so that the final Cl- is 1M. Now the measured emf of the cell is 0.222 V.
The Ksp of AgCl would be :
In the third period of the periodic table the element having smallest size is
Gibbs free energy change for a cell reaction is positive what does it indicates?
An element has configuration 4d55s2. The element belongs to