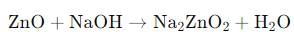Class 10 Exam > Class 10 Tests > Science Class 10 > Test: Important Chemical Compounds - 2 - Class 10 MCQ
Test: Important Chemical Compounds - 2 - Class 10 MCQ
Test Description
15 Questions MCQ Test Science Class 10 - Test: Important Chemical Compounds - 2
Test: Important Chemical Compounds - 2 for Class 10 2025 is part of Science Class 10 preparation. The Test: Important Chemical Compounds - 2 questions and answers have been
prepared according to the Class 10 exam syllabus.The Test: Important Chemical Compounds - 2 MCQs are made for Class 10 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Important Chemical Compounds - 2 below.
Solutions of Test: Important Chemical Compounds - 2 questions in English are available as part of our Science Class 10 for Class 10 & Test: Important Chemical Compounds - 2 solutions in
Hindi for Science Class 10 course. Download more important topics, notes, lectures and mock
test series for Class 10 Exam by signing up for free. Attempt Test: Important Chemical Compounds - 2 | 15 questions in 15 minutes | Mock test for Class 10 preparation | Free important questions MCQ to study Science Class 10 for Class 10 Exam | Download free PDF with solutions
Test: Important Chemical Compounds - 2 - Question 1
What is the defining characteristic of salts?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 1
Test: Important Chemical Compounds - 2 - Question 2
Which compound can be created from common salt, sodium chloride (NaCl), and is commonly known as caustic soda?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 2
Test: Important Chemical Compounds - 2 - Question 3
In the family of salts, when salts have the same cation or anion, how are they categorized?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 3
Test: Important Chemical Compounds - 2 - Question 4
What is the primary purpose of the chlor-alkali process?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 4
Test: Important Chemical Compounds - 2 - Question 5
What is the common characteristic shared by all acids and bases?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 5
Test: Important Chemical Compounds - 2 - Question 6
When acids are dissolved in water, what ions do they produce?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 6
Test: Important Chemical Compounds - 2 - Question 7
What happens when a base is dissolved in water?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 7
Test: Important Chemical Compounds - 2 - Question 8
Why is it recommended to add acid to water when diluting acids instead of adding water to acid?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 8
Test: Important Chemical Compounds - 2 - Question 9
What does a pH value of 7 indicate about a solution?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 9
Test: Important Chemical Compounds - 2 - Question 10
How is the strength of an acid or base estimated using a universal indicator?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 10
Test: Important Chemical Compounds - 2 - Question 11
What is the general chemical equation for the reaction between a metallic oxide and an acid?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 11
Test: Important Chemical Compounds - 2 - Question 12
How do metallic oxides generally react with bases?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 12
Test: Important Chemical Compounds - 2 - Question 13
What is the product of the reaction between ZnO and sodium hydroxide (NaOH)?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 13
Test: Important Chemical Compounds - 2 - Question 14
When a non-metallic oxide reacts with a base, what is the general outcome in terms of products?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 14
Test: Important Chemical Compounds - 2 - Question 15
Which type of reaction occurs when a base reacts with a non-metallic oxide?
Detailed Solution for Test: Important Chemical Compounds - 2 - Question 15
|
80 videos|512 docs|74 tests
|
Information about Test: Important Chemical Compounds - 2 Page
In this test you can find the Exam questions for Test: Important Chemical Compounds - 2 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Important Chemical Compounds - 2, EduRev gives you an ample number of Online tests for practice