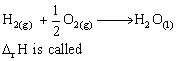KVS PGT Chemistry Mock Test - 6 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test KVS PGT Exam Mock Test Series 2025 - KVS PGT Chemistry Mock Test - 6
The following sentence has been broken into four parts with an error in one part. Identify that part and mark it as your answer. If there are no errors in any of the given parts, mark option 4 or ‘No error’ as your answer.
Q. The metro rail management apologized (1)/ for the inconsistency it had caused (2)/ to all the commuters. (3)/ No error (4).
The following sentence has been broken into four parts with an error in one part. Identify that part and mark it as your answer. If there are no errors in any of the given parts, mark option 4 or ‘No error’ as your answer.
Kajal was sitting within (1)/ between Prachi and Isha (2)/ at the party that night. (3)/ No error (4).
In the following question, an idiomatic expression and its four possible meanings are given. Find out the correct meaning of the idiom.
A drop in the bucket.
In the following question, an idiomatic expression and its four possible meanings are given. Find out the correct meaning of the idiom.
Q. M.F. Hussain's works always paint a thousand words.
A word with letters jumbled has been given. Choose the correct order of letters which are required to form the correct word.
Jumbled word: PTIOISCL
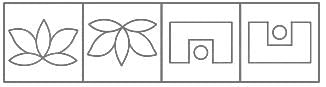
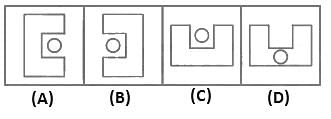
Which figure from the answer figures will replace the question mark (?) in the problem figures?
Problem Figures:
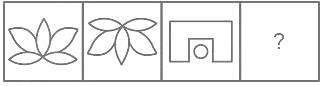
Answer Figures:
Which of the statements given below is NOT TRUE for corporal punishment?
Which of the following statements is true, according to the principle of unequal development rate ?
______health committee was set up to assess the health condition of India.
[Ti (H2O)6]3+ absorbs green and yellow region part of visible light. Then the transmitted colour of the compound is
The conditions favourable for the reaction :
2SO2(g)+O2(g) 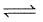 2SO3(g) ; ΔH° = -198 kJ are :
2SO3(g) ; ΔH° = -198 kJ are :
In the electrolysis of aqueous sodium chloride solution, two types of reactions can take place at anode :
I. 2Cl- (aq) → Cl2(g) +2e-
II. 2H2O(l)g → O2 (g) + 4H+(aq) + 4e-
Select the correct statement(s) about these.
How many atoms of hydrogen are in 67.2 L of H2 at STP?
The energies of activation for forward and reverse reactions for A2 + B2 2AB are 180 kJ mol–1 and 200 kJ mol–1 respectively. The presence of a catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol–1. The enthalpy change of the reaction (A2 + B2 → 2AB) in the presence of catalyst will be (in kJ mol–1) –
[AIEEE 2007]
Chemical species present in the environment are either naturally occurring or generated by human activities. Their interrelation with the surroundings is called:
Which of the following statements are not true for hydrogen?
Which of the following elements are called representative elements?
Bond length of H— Cl is 1.2476 Thus, its dipole moment is
A solution was made by dissolving 2 g of a solute in 100 g of acetone. The solution boiled at 56.95° C. The boiling point of pure acetone is 55.95° C, and the Kb =1.71°C/m. What is the molecular weight of the solute?
The most and least reactive electrophiles respectively in a SN1 reaction are
In which of the following compounds the carbon marked with asterisk is expected to have greatest positive charge?