Test: Class 8 General Science NCERT Based - 2 - UPSC MCQ
25 Questions MCQ Test - Test: Class 8 General Science NCERT Based - 2
Consider the following pairs:
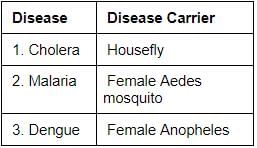
Which of the pairs given above is/are correctly matched?

Consider the following pairs:
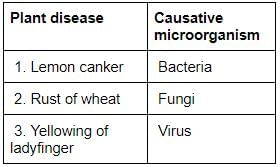
Which of the pairs given above is/are correctly matched?

Consider the following statements regarding food preservatives.
1. Sodium Benzoate and Sodium Metabisulfite are common food preservatives.
2. Salt, sugar, edible oil and vinegar are used as food preservatives.
Which of the statements given above is/are correct?
Consider the following statements regarding pasteurised milk.
1. Pasteurised milk can be consumed without boiling, because it is free of microorganisms.
2. In order to pasteurise the milk, it is first heated to a temperature of 70 degree celsius, for 15-20 minutes; and then cooled rapidly; and packaged.
Which of the statements given above is/are correct?
Which of the following statements is not correct, in regard to nitrogen cycle?
Consider the following:
1. Cotton
2. PET
3. Plastic
Which of the above is/are example(s) of natural polymers?
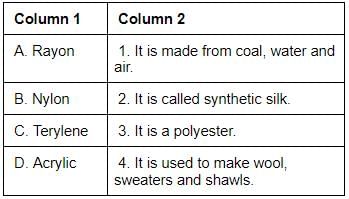
Match the items of column 1 with column 2 and select the correct answer using the code given below.
Uniforms of firemen have a coating to make them flame resistant. Which of the following is used to make that coat?
Which of the following metals remains in liquid state at room temperature?
If copper utensils are exposed to moist air for a long time, they develop a green layer over them. Which of the following is that green substance?
Which of the following is a highly reactive metal which catches fire when exposed to air?
Name the synthetic polymer that resembles natural rubber?
Name the polymer that can have strong intermolecular forces?
Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
1. Good thermal conductivity
2. Good electrical conductivity
3. Ductility
4. High melting point
Which of the following represent the correct order of decreasing reactivity?
Where was the first oil well of the world drilled?
Naphthalene balls, used to combat moths and other pests, are obtained from which of the following?
Consider the following statements regarding combustion and ignition temperature?
1. Oxygen is required for the combustion of materials.Heat and light are given out in this process.
2. The lowest temperature at which a substance (fuel) catches fire is called its ignition temperature.
3. The combustion of phosphorus occurs at room temperature, in the presence of air.
Which of the statements given above is/are correct?
Consider the following statements in context of safety matches:
1. A mixture of Antimony Trisulphide and Potassium Chlorate is applied on the heads of matchsticks.
2. The rubbing surface has powdered glass and a little white phosphorus.
Which of the statements given above is/are correct?
The job of a fire extinguisher is to cut off the supply of air, or to bring down the temperature of the fuel, or both. Consider the following statements in the context of cutting off the supply of air:
1. Water vapours, formed due to water being poured on the flame, surround the combustible material, helping in cutting off the supply of air.
2. CO2, being heavier than oxygen, covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off, the fire is controlled.
Which of the statements given above is/are correct?
Of the following statements, which of the following is/are correct regarding candle flames?
1. Unburnt carbon particles are present in the luminous zone of the flame.
2. The non-luminous zone of the flame has a highest temperature.
Consider the following statements:
1. Incomplete combustion of fuels produce the poisonous gas, carbon dioxide.
2. Excessive proportion of carbon dioxide in the environment results in global warming.
3. Oxides of sulfur and nitrogen mix with rain, and result in acid rain.
Which of the above statements/statements is/are correct?
Assertion (A): In India, 98% of the coal is found in Gondwana rocks of the Moran region.
Reason (R): The main regions of Gondwana rocks are found in West Bengal, Jharkhand and Odisha.
Codes:
The major constituent of biogas is:
Carbonisation is a process in which














