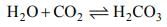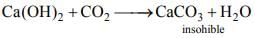Test: Chemistry - 3 - UPSC MCQ
20 Questions MCQ Test - Test: Chemistry - 3
Match List-I with List-II and select the correct answer from the codes given below:
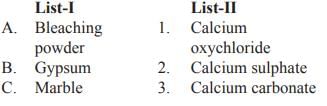



Match List-I with List-II and select the correct answer from the codes given below:
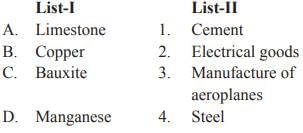



Match List-I with List-II and select the correct answer from the codes given below:

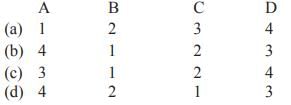
Consider the following statements and select the correct code.
Assertion (A): The main constituent of the liquefied petroleum gas is methane.
Reason (R): Methane can be used directly for burning in homes and factories where it can be supplied through pipelines.
Which one among the following statements regarding the properties of mixtures and compounds is not correct?
Which of the following pairs is/are correctly matched?
1. Isotopes : Atoms with same atomic number but different atomic mass.
2. Isobars : Atoms with same number of neutrons but different atomic number.
3. Isotones : Atoms with same mass number but different atomic number.
Q. Select the correct answer using the codes given below :
Match List–I with List–II and select the correct answer using the code given below:
List- I List-II


Consider the following statements regarding diamond:
1. It is an allotrope of silicon.
2. It is a bad conductor of heat and electricity.
3. It is the hardest substance.
4. It burns to produce carbon dioxide.
Q. Which of the statements given above are correct?
Following statements are made in connection with carbon dioxide (CO2)
1. CO2 is a poisonous gas.
2. CO2 is an acidic oxide.
3. CO2 turns limewater milky.
Which of the statements given above is/are correct?
Which of the following statements about diamond are correct?
1. It is used as a gem in jewellery because of its ability to reflect light.
2. It is good conductor of electricity.
3. It is used for cutting glass, marble stones and other hard materials.
4. It is used for drilling of rocks.
Q. Select the correct answer using the codes given below :
Consider the following statements :
1. Diamond is hard and graphite is soft.
2. Diamond is soft and graphite is hard.
3. Diamond is a bad conductor but graphite is a good conductor.
4. Diamond is a good conductor but graphite is a bad conductor.
Q. Which of the statements given above is/are correct ?
Consider the following statements:
Nitrogen is an essential constituent of
1. soils
2. animals
3. plants
Q. Which of the statements given above is/are correct ?
When iron is left exposed in open air, it gets rusted.
Which constituent(s) of air is /are responsible for rusting iron?
1. Oxygen gas present in air
2. Moisture present in air
3. Carbon dioxide gas present in air
Q. Select the correct answer using the codes given below :
Which of the statements given below is/are correct?
Permanent hardness of water is due to the presence of soluble.
1. chloride of calcium
2. bicarbonate of calcium
3. sulphate of magnesium
4. bicarbonate of magnesium
Q. Select the correct answer using the codes given below.
Consider the following statements :
1. An alloy is a mixture of two or more metals.
2. An alloy is a mixture of a metal or metals with a non-metal.
Q. Which of the statements given above is/are correct ?
Match List–I with List–II and select the correct answer using the codes given below :


Consider the following statements and select the correct code.
Assertion (A) : In the periodic table of chemical elements, electron affinity is always found to increase from top to bottom in a group
Reason (R) : In a group, the atomic radii generally increase from top to bottom.
What happened when a hard boiled egg after shelling is immersed in saturated brine?
Consider the following statements : The purpose of adding sodium sulphate and sodium silicate to washing powder is -
1. To keep washing powder dry
2. To maintain the alkalinity of the powder
Q. which of these statements is/are correct ?