Test: Types Of Displacement Reactions - UPSC MCQ
15 Questions MCQ Test Science & Technology for UPSC CSE - Test: Types Of Displacement Reactions
In a given reaction, barium hydroxide reacts with ammonium chloride to form product X and Y. Identify X and Y.
Ba(OH)₂ + 2NH₄Cl → X + Y
Ba(OH)₂ + 2NH₄Cl → X + Y
Which of the following is an example of displacement reaction?
Identify the reaction:
AgNO3(aq) + KCl(aq) → AgCl↓ + KNO3(aq)
The reaction, Zn + 2HCl → ZnCl2 + H2 is an example of:
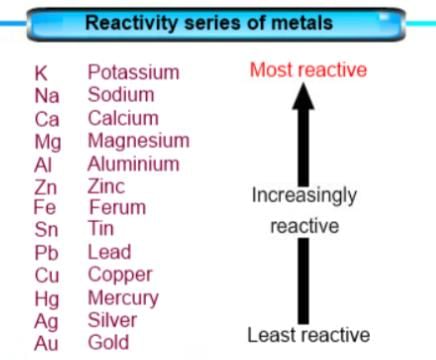
Which metallic spoon can be used to stir lead nitrate solution?
Which of the following metal comes below copper in reactivity series?
The reaction between lead nitrate and potassium iodide is an example of _______ .
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:
Which of the following metals comes above zinc in reactivity series?
The given reaction indicates that:
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
The colours of aqueous solutions of CuSO4 and FeSO4 as observed in the laboratory are:
A strip of copper was placed in a beaker containing zinc sulphate solution. On observing the strip next day, it was noticed that:
Which form of energy is responsible for the decomposition reactions ?
Why does an iron nail become brownish in color and the blue color of copper sulfate solution fades?
Assertion (A): The iron nail becomes brownish in color and the blue color of copper sulfate solution fades.
Reason (R): In the reaction Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s), iron displaces copper in the copper sulfate solution.
|
114 videos|428 docs|209 tests
|